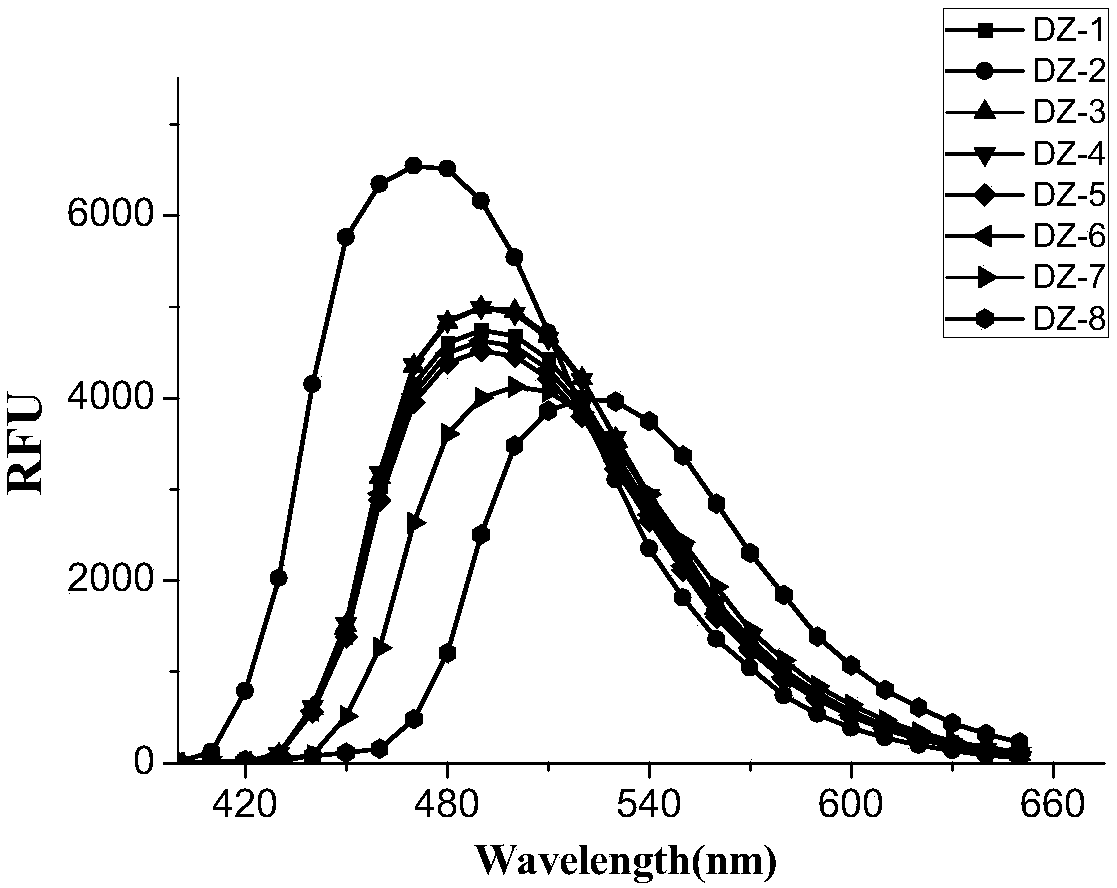
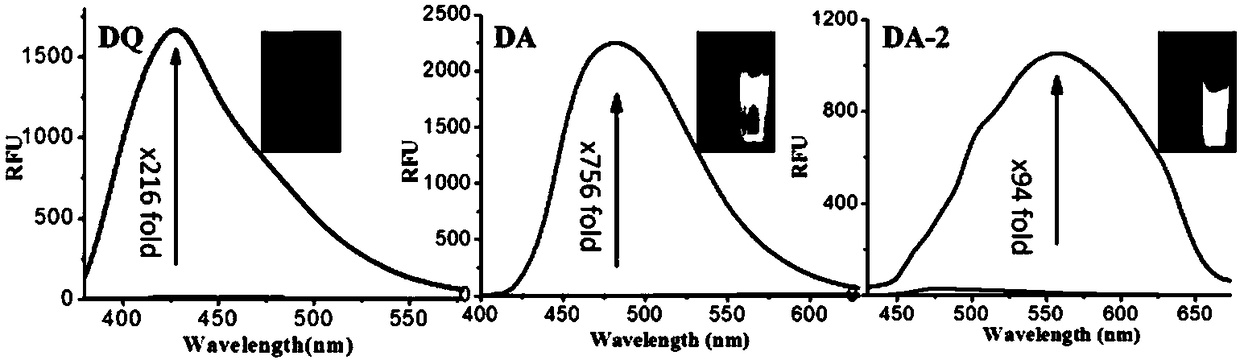
Fluorescent dye with fluorescence opening property and preparation method and application thereof
A technology of fluorescent dyes and properties, applied in the field of fluorescent dyes, can solve the problems of lack of synthesis methods and hinder the development of fluorescent azide probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Synthesis of 5-azido-8-aminoquinoline (DA)
[0063]
[0064] In a 100 mL single-necked flask, 8-benzamide quinoline (10 mmol) and KOH (20 mmol) were dissolved in an appropriate amount of methanol, and the resulting mixture was heated at 80°C for 12 hours and cooled to room temperature. The reaction solution was treated with CH 2 Cl 2 Wash three times, collect CH 2 Cl 2 The solution was washed three times with saturated NaCl aqueous solution and then Na 2 SO 4 Dry and remove the solvent by rotary evaporation to obtain a crude product. The crude product was further purified through a silica gel column using a mixed solvent of PE / EtOAc (v / v; 2:1) as the eluent to obtain the product DA (yield 75%) as a white solid.
[0065] 1H NMR (500MHz, CDCl 3 )δ8.11(s,1H), 8.05(d,J=8.6Hz,1H), 7.70(d,J=16.3Hz,2H), 7.64(dd,J=13.1,8.0Hz,3H),7.44– 7.40(m,2H), 7.36–7.28(m,2H), 7.14(d,J=8.0Hz,1H), 6.94(dd,J=7.5,0.9Hz,1H), 5.08(s,2H);
[0066] 13C NMR(126MHz, CDCl 3 )δ154.23,148.06,135.51...
Embodiment 2
[0067] Example 2: Synthesis of dye precursor compound 2 (DQ)
[0068]
[0069] Add 8-aminoquinoline (10mmol) and triethylamine (15mmol) into a 100mL single-neck flask, and dissolve in CH 2 Cl 2 (30mL). After stirring for 5 minutes at room temperature, the reaction solution was cooled in an ice bath. Add acid chloride (11 mmol) dropwise. The reaction solution was then stirred overnight, then the mixture was filtered through a pad of Celite, and the residue was washed with CH 2 Cl 2 (25mL) Wash the collected CH 2 Cl 2 1M NaHCO for solution 3 Wash with aqueous solution three times. Collect the organic layer and use anhydrous Na 2 SO 4 dry. The solvent was removed by rotary evaporation, and the obtained crude product was purified by a silica gel column with PE / EtOAc (v / v; 10:1) mixed solvent to obtain pure product 2 (yield 80%).
[0070] 1H NMR (500MHz, CDCl 3 )δ10.61(s,1H), 8.90(dd,J=7.6,1.1Hz, 1H), 8.68(dd,J=4.2,1.6Hz,1H), 8.03(dd,J=3.7,2.1Hz,2H ), 7.95 (dd, J = 8.2, 1.6 Hz, 1H),...
Embodiment 3
[0072] Example 3: Synthesis of dye DA-1
[0073]
[0074] Add 6-styrene-5azide-8aminoquinoline (10mmol) and KOH (20mmol) to a 250mL round bottom flask, and dissolve in 100mL methanol. The resulting mixture was heated at 80°C for 12 hours and cooled to room temperature. The mixture was removed by rotary evaporation, and the residue was replaced with CH 2 Cl 2 Wash three times. CH collected 2 Cl 2 The solution was washed three times with aqueous NaCl solution. After that, collect the organic layer and use Na 2 SO 4 dry. The solvent was removed by rotary evaporation. Then the crude product was purified by passing through a silica gel column using a PE / EtOAc (v / v: 2:1) mixed solvent as an eluent. And provide the product DA-1 (yield 75%) yellow solid.
[0075] 1H NMR (500MHz, CDCl 3 ): δ=5.92(s,2H), 7.05(d,J=2.5Hz, 1H), 7.29-7.41(m,5H), 7.60-7.63(m,4H), 7.99(dd,J1=14.0Hz, J2=8.5Hz, 2H);
[0076] 13C NMR(125MHz, CDCl 3 ): δ=105.2, 119.5, 122.2, 127.0, 128.2, 128.3, 128.7, 129.0, 130....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com