Drug delivery carrier modified by dihydroartemisinin and its application in pharmacy
A technology of dihydroartemisinin and drugs, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of not being able to fully exert the design targeting function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of carrier PPD modified by dihydroartemisinin:
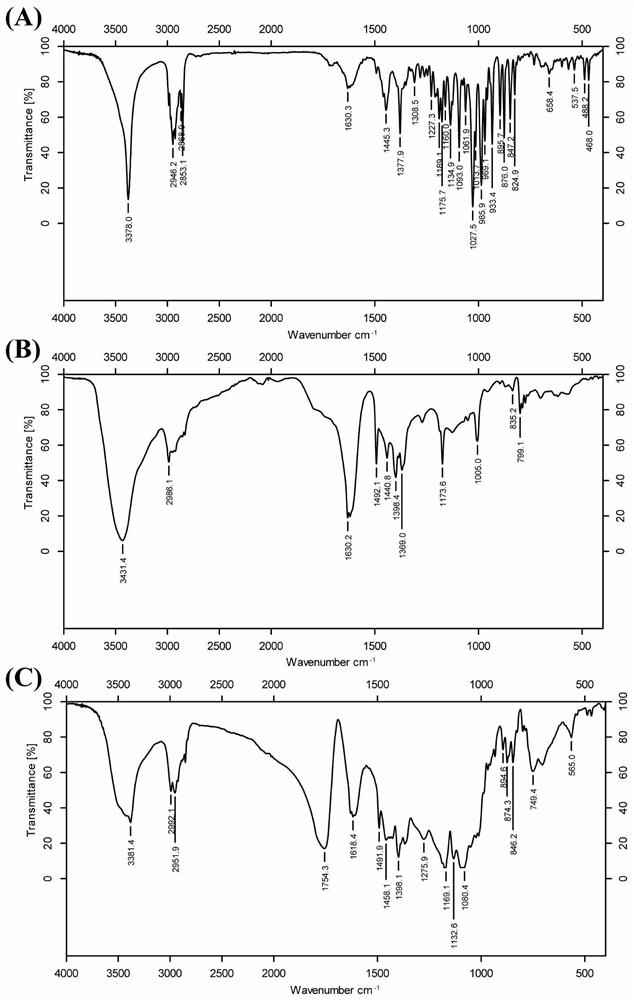
[0046] Add polyethylene glycol (HO-PEG2000-COOH) into the three-necked flask, stir for 2 hours under reduced pressure nitrogen in an oil bath at 140°C, then add an equal amount of glycolide, lactide and an appropriate amount of stannous octoate The three-necked flask was refluxed for 10 h under the condition of an oil bath at 140° C., and PLGA-PEG2000-COOH was obtained by precipitation and extraction with ether. The above PLGA-PEG2000-COOH and dihydroartemisinin were reacted for 48 hours under the catalysis of EDCI and HObt to obtain PPD.
[0047]
[0048] The molecular weight of PLGA in the step is 8000, but it is not limited thereto. The PLGA of the present invention can also be PLGA modified with a carboxyl group at one end, but it is not limited to the above substances. The molecular weight of PLGA can be in the range of 8000-38000.
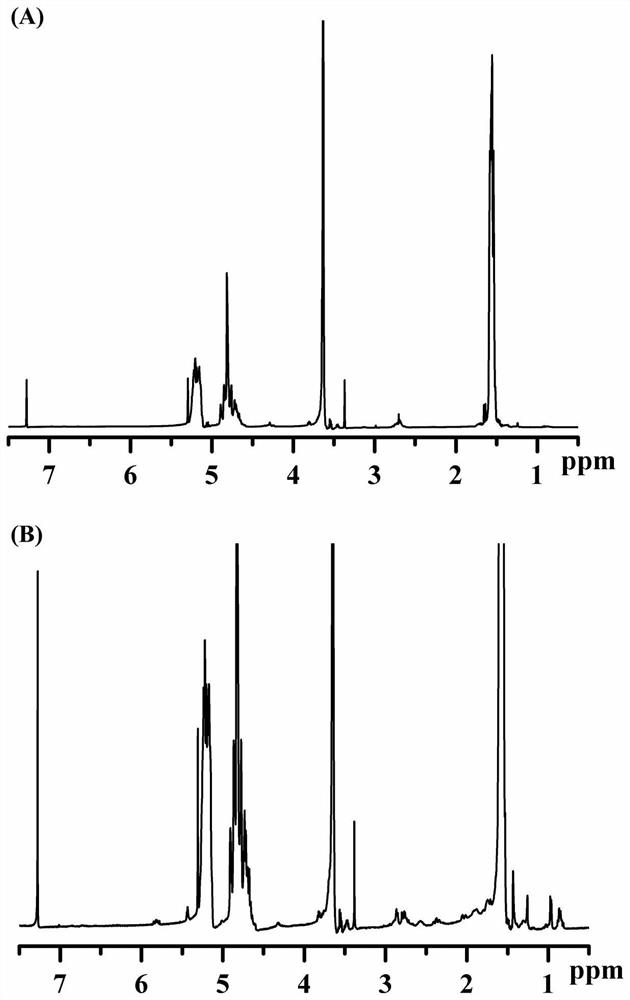
[0049] Determination by NMR 1 H-NMR hydrogen spectrum determines t...
Embodiment 2
[0051] Preparation of Docetaxel-loaded Nanoparticles by Emulsion Solvent Evaporation
[0052] Weigh 1 mg of docetaxel or 0.1 mg of coumarin 6, dissolve it in an appropriate amount of dichloromethane, add 20 mg of the PPD prepared in Example 1, add 5 mL of deionized water containing 0.5% PVA, ultrasonicate the probe at 300 W for 5 min, and use centrifugation Remove unwrapped medication. The preparation method of PP nanoparticles is similar, just replace PPD with PP.
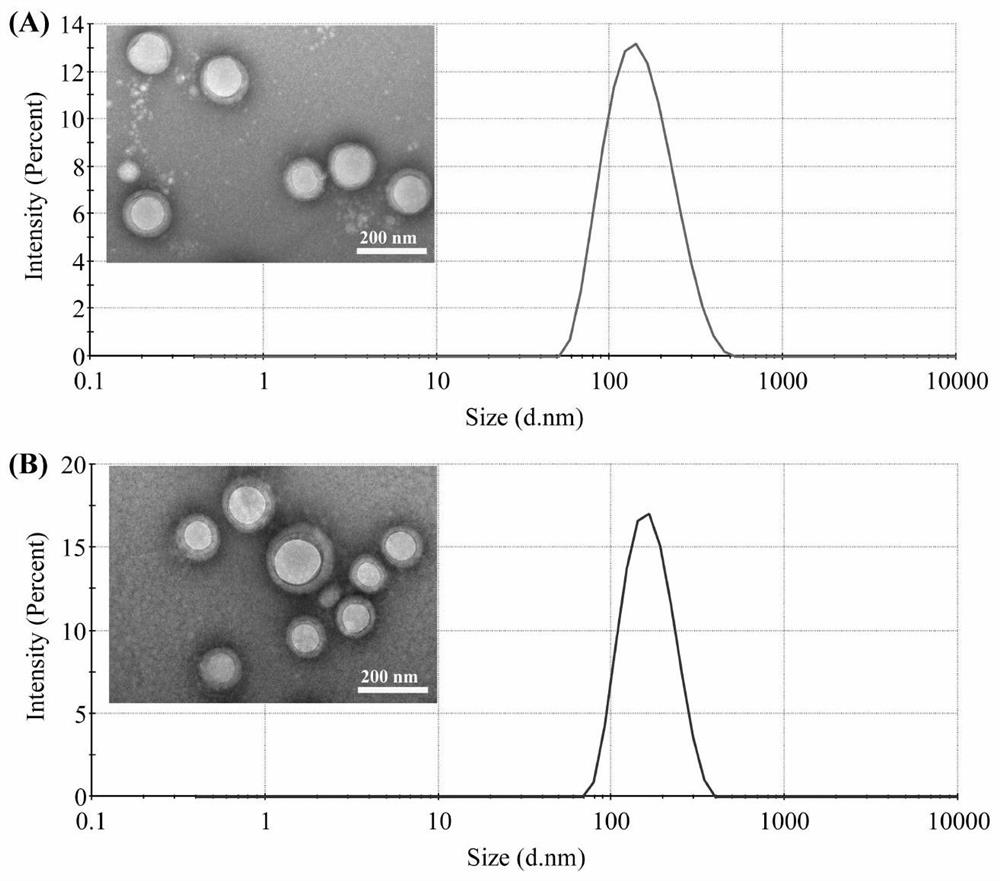
[0053] The nanoparticles prepared in Example 2 were measured by dynamic light scattering and transmission electron microscopy for their particle size and shape. The result is as image 3 , the particle size of the nanoparticles is about 150nm, and the particle size distribution is narrow; the transmission electron microscope shows that the drug-loaded nanoparticles are spherical with uniform particle size.
Embodiment 3
[0055] Preparation of DHA-PEG-Stearate-modified liposomes loaded with docetaxel or coumarin 6 by film dispersion method
[0056] Weigh 1 mg of docetaxel or 0.1 mg of coumarin 6, dissolve in an appropriate amount of dichloromethane, add 2 mg of DHA-PEG-Stearate (dihydroartemisinin-polyethylene glycol-stearate) prepared in Example 2 vehicle), and 30 mg of soybean lecithin and 1 mg of cholesterol. Spin dry to form a film, add 2 mL of deionized water for hydration for 30 min, sonicate the probe at 300 W for 5 min, and remove uncoated drugs by microcolumn centrifugation.
[0057] The liposomes prepared in Example 3 were determined by dynamic light scattering and transmission electron microscopy to determine the particle size and shape of the liposomes. The result is as Figure 4 , 5 , the particle size of the liposome is about 130nm, and the particle size distribution is narrow; the transmission electron microscope shows that the drug-loaded liposome is spherical with uniform pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com