Purification method of difluprednate intermediate
A technique of difluprednate and a purification method, which is applied in the field of purification of organic matter, can solve problems such as high cost, low production capacity, and difficult large-scale production, and achieve the effects of saving cost, reducing impurity content, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
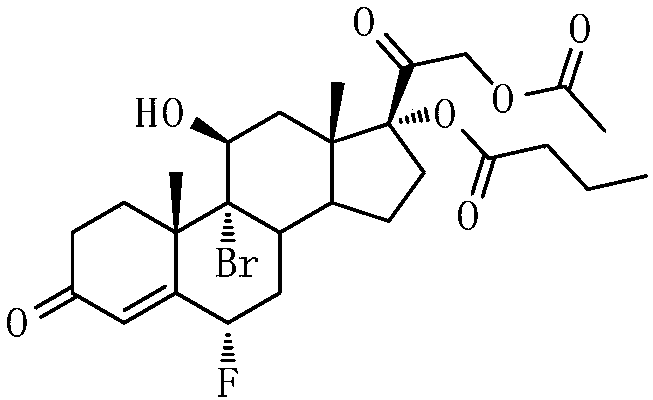
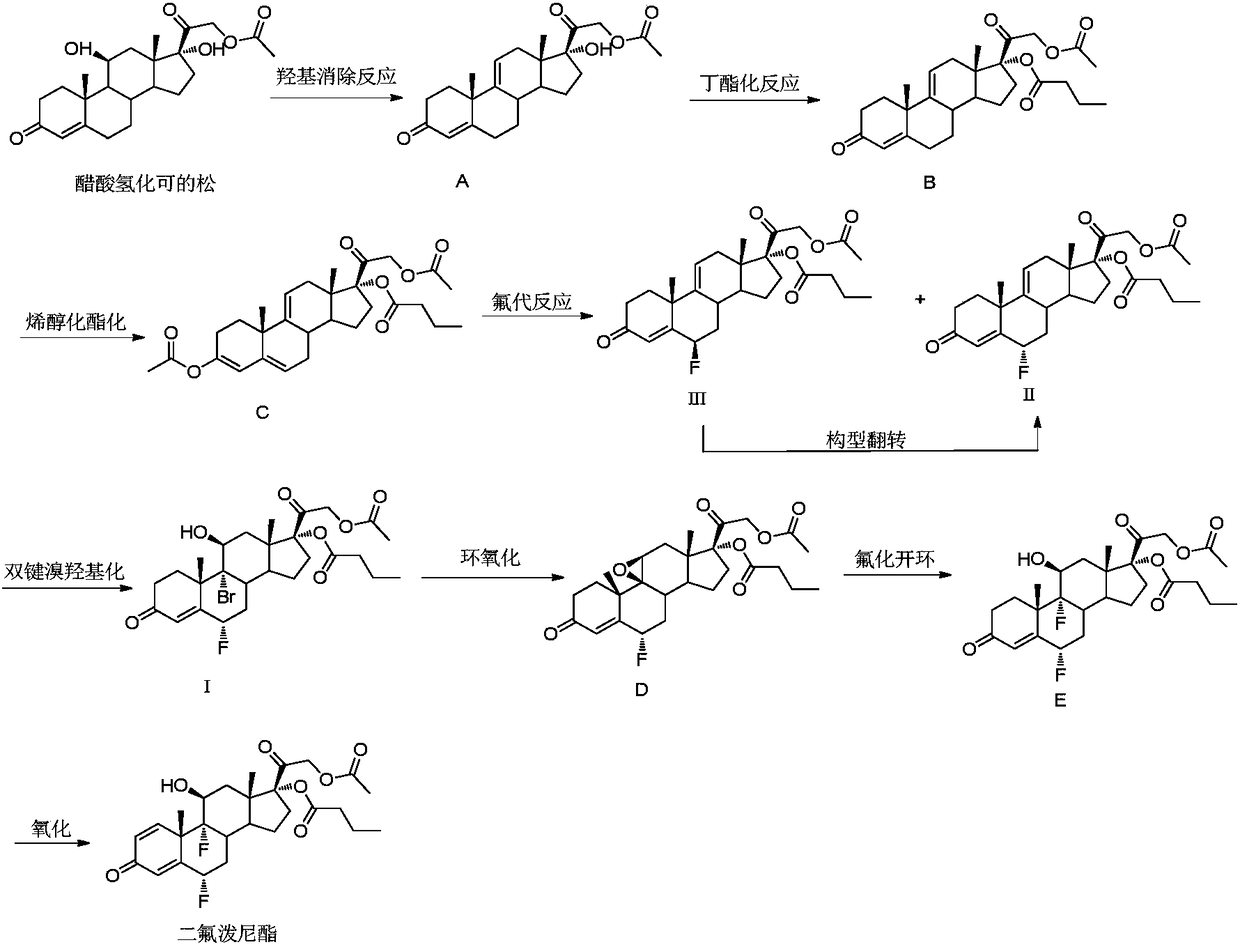
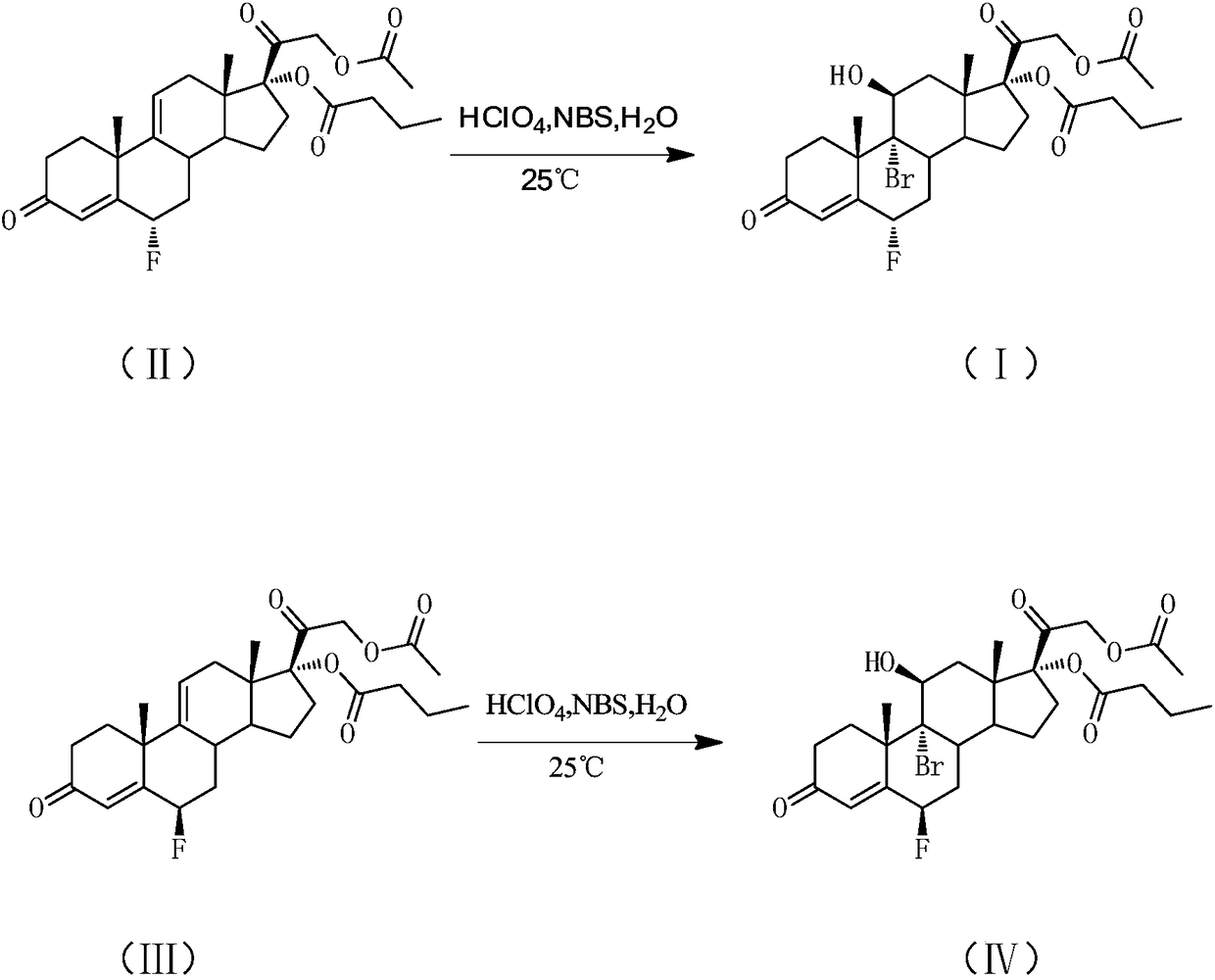
[0056] An embodiment of the purification method of the compound shown in formula I, the purification method of the compound shown in formula I described in this embodiment is: add 1.00g Na 2 SO 3 The solid was rotary evaporated at T=35°C to obtain a yellow solid, the first crude product; 4.0mL MTBE was added to the first crude product and stirred at 0°C for 30min, filtered, washed with 1.0mL MTBE, and washed with 10.0mL water to obtain White solid, the white solid was dried at T=50°C for 5h, namely the second crude product; adding isopropyl acetate and n-hexane to the second crude product to prepare a saturated solution at T=90°C, the isopropyl acetate The volume ratio to n-hexane is: isopropyl acetate: n-hexane = 7:1, then cool down to 25°C to crystallize, filter to obtain a white solid, and dry the obtained solid at T=50°C to obtain formula I compound.
[0057] The reaction yield of the obtained compound represented by formula I was 75%. According to the results of HPLC, t...
Embodiment 2
[0059] An embodiment of the purification method of the compound shown in formula I, the purification method of the compound shown in formula I described in this embodiment is: add 1.00g Na 2 SO 3 Solid, the solvent was recovered by rotary evaporation at T=35°C to obtain a yellow solid, the first crude product; add 4.0mL MTBE to the first crude product, stir at 0°C for 30min, filter, wash with 1.0mL MTBE, and wash with 10.0mL water After washing, a white solid was obtained. The white solid was dried at T=50° C. for 5 h, namely the compound represented by formula I.
[0060] The reaction yield of the obtained compound represented by formula I was 90%. According to the results of HPLC, the purity of the obtained compound represented by formula I was above 90.0%, and the maximum single heterogeneity was less than 1.0%.
Embodiment 3
[0062] An embodiment of the purification method of the compound shown in formula I, the purification method of the compound shown in formula I described in this embodiment is: add 1.00g FeCl to 1.00g of the solution containing the compound shown in formula I 2 Solid, the solvent was recovered by rotary evaporation at T=35°C to obtain a yellow solid, the first crude product; add 4.0mL MTBE to the first crude product, stir at 0°C for 30min, filter, wash with 1.0mL MTBE, and wash with 10.0mL water After washing, a white solid was obtained. The white solid was dried at T=50° C. for 5 h, namely the compound represented by formula I.
[0063] The reaction yield of the obtained compound represented by formula I was 60%. According to the results of HPLC, the purity of the obtained compound represented by formula I was above 85.0%, and the maximum single heterogeneity was less than 2.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com