Human encephalitis B virus antibody solid phase competition ELISA detection kit, and preparation method and detection method thereof
A technology of Japanese encephalitis virus and detection kit, which is applied in the direction of biological testing, measuring devices, material inspection products, etc., and can solve the problems of inability to detect the antibody titer of the tested sample, inability to judge the infection or the recovery status of the infection period, There are no problems such as detection of antibody titer, and the effects of eliminating species source differences, low cost, and easy operation are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Preparation of Human Japanese Encephalitis Virus Antigen
[0044] The cultivated Vero cells, when the cell growth density of Vero (African green monkey kidney cells: commonly used cells for virus cultivation) reached about 80%, the human Japanese encephalitis virus (10 5 TCID 50 ), after melting on ice, make a 5-fold dilution with OPTI-MEM, take 2.5mL to infect 75cm 2 Incubate the cells in the cell bottle at 37°C for 1 hour, shake gently 2-3 times every 15 minutes, then discard the virus infection solution, add 10mL OPTI-MEM, and finally place at 37°C, 5% CO 2 cultured in a constant temperature incubator. After 3 days, the cells were damaged. After the diseased cells floated, the cell bottle was placed at -80°C, freeze-thawed three times, and then centrifuged at 4°C, 8000rpm for 30 minutes to remove cell debris, and then the supernatant was placed at 4°C, Centrifuge at 35000rpm (horizontal rotor) for 3 hours, discard the supernatant, suspend the precipitate ...
Embodiment 2
[0045] Embodiment 2. Preparation of serum
[0046] (1) Preparation of human Japanese encephalitis virus positive serum
[0047] In this study, positive serum of human Japanese encephalitis virus was used as the serum samples of infected patients obtained from clinical diagnosis and treatment. Part of it is hyperimmune serum obtained from a small amount of blood samples obtained from volunteers vaccinated against Japanese encephalitis virus. After the serum is prepared, it is stored in -80°C.
[0048] (2) Preparation of monkey anti-human Japanese encephalitis virus serum
[0049] Select healthy male monkeys weighing 20-25 kg, emulsify the ultra-purified human Japanese encephalitis virus with an equal volume of complete Freund's adjuvant, and inject it subcutaneously at multiple points on the back, with a dose of 3 mg / monkey; 15 days after the first immunization , human Japanese encephalitis virus was emulsified with an equal volume of incomplete Freund's adjuvant, and the mon...
Embodiment 3
[0050] Embodiment 3. Optimization of reaction conditions
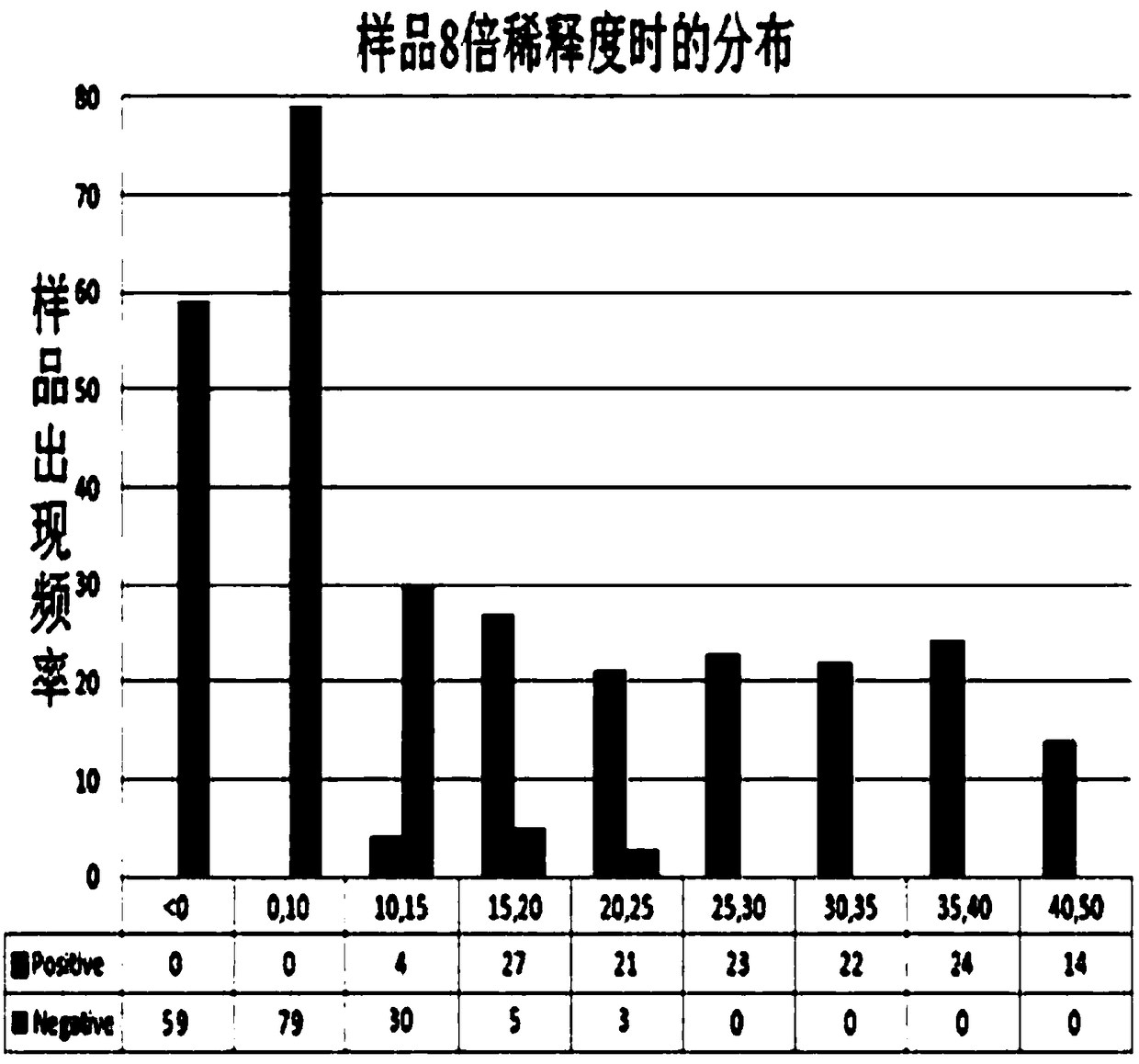
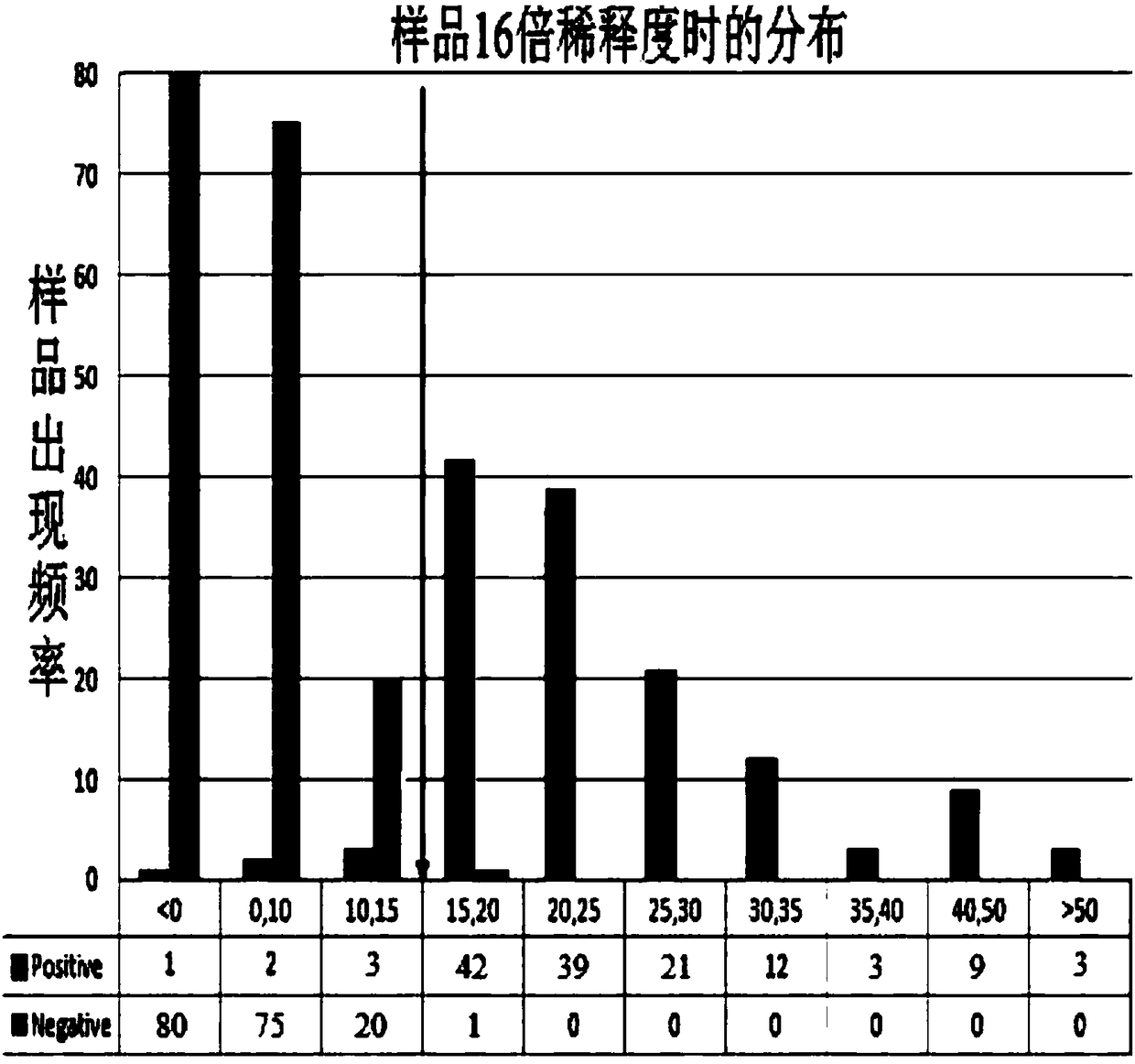
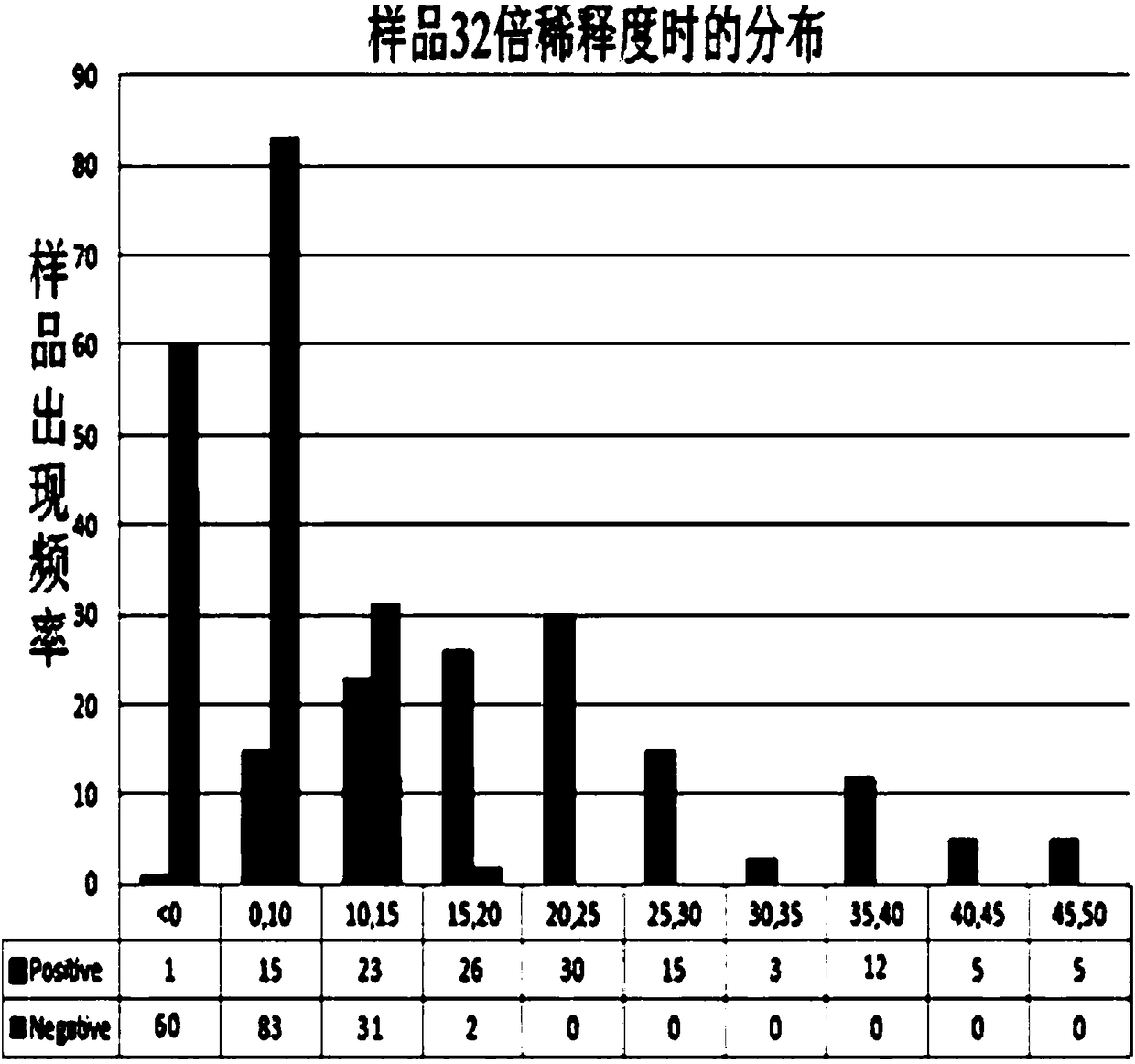
[0051] The coating concentration of the inactivated antigen of human Japanese encephalitis virus, the dilution ratio of monkey anti-human Japanese encephalitis virus positive serum, the dilution ratio of HRP-labeled anti-monkey IgG secondary antibody and the concentration of blocking solution were optimized by matrix method. The OD ratio of negative and positive serum is the largest, and when the OD value of the complete monkey competition serum well (this well is only added with diluted monkey anti-human Japanese encephalitis virus positive serum and the same amount of PBST) is about 1.5, determine the reaction conditions: coating ELISA plate (50 μL / well), the antigen concentration is 10 ng / μL, blocked with 2% BSA, 37 ° C, 1 hour (100 μL / well); monkey competition serum was diluted 1:1000 (50 μL / well), HRP-labeled The anti-monkey IgG enzyme-labeled secondary antibody was diluted 1:3000 (50 μL / well).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com