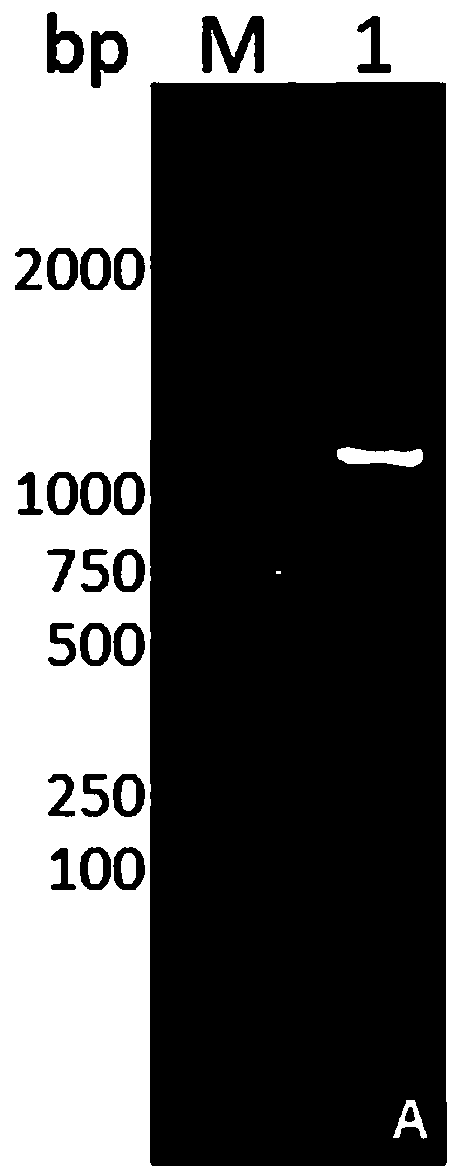
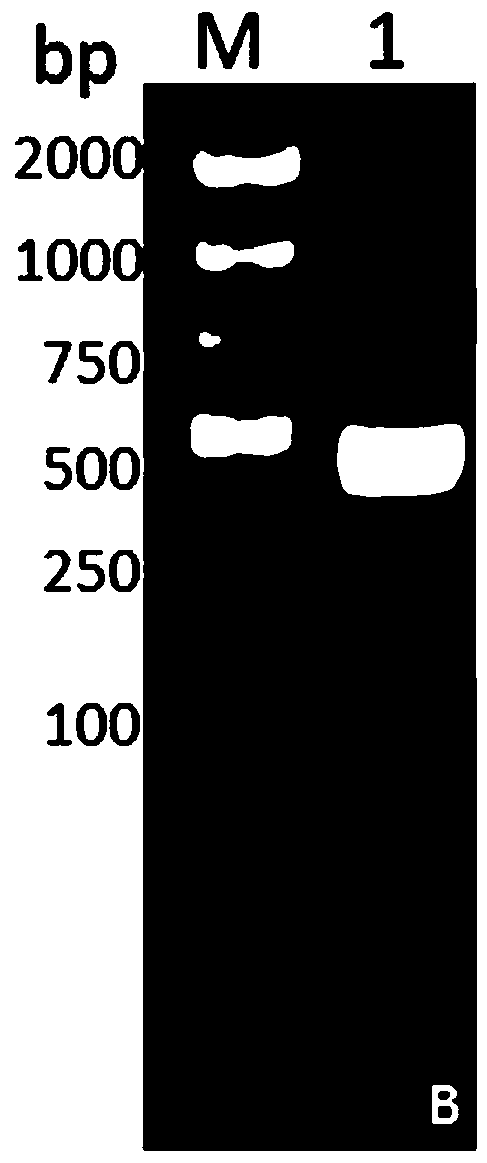
DNA vaccine for preventing and treating periodontitis as well as preparation method thereof
A technology of DNA vaccine and gingivalin, which is applied in the field of preparation, DNA vaccine, and prevention and treatment of periodontitis, can solve problems such as difficult recovery and periodontal disease damage to periodontal tissue, and achieve low production cost, easy promotion, and method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Construction of DNA vaccine for preventing and treating periodontitis—pVAX1-HA2-fimA
[0044] Step 1, cultivation of Porphyromonas gingivalis W83 type
[0045] The frozen Porphyromonas gingivalis type W83 (preserved by the Key Laboratory of Oral Diseases Research in Guizhou Province) was streaked and cultured on BHI blood agar culture plates (purchased from Beijing Suo Laibao Biotechnology Co., Ltd.). The conditions are 37°C anaerobic box (80%N 2 , 10%H 2 , 10% CO 2 ), protected from light for 4-7 days, observed that the black colony grew to about 1 mm, and picked the single colony grown on the plate to inoculate to the BHI liquid medium (commercial flat medium, purchased from Shanghai Hengyuan Biotechnology Co., Ltd. ), cultured in an anaerobic incubator for 1-2 days, it was observed that the liquid became turbid, and a small amount of bacteria precipitated, and the bacteria liquid was collected for subsequent experiments.
[0046] Step 2. Obtaining the ...
Embodiment 2
[0127] Example 2: Experimental Study on SD Rats Immunized by Periodontitis DNA Vaccine
[0128] 1. Mass preparation of plasmid vaccines
[0129] Purify and recover pVAX1-HA2-fimA and empty vector pVAX1 with Promega plasmid extraction kit.
[0130] 2. Eighteen 6-8 week male SD rats were randomly divided into 3 groups, experimental group: group A, control group: group B, C, 6 rats in each group, as follows:
[0131] Group A: sample group pVAX1-HA2-fimA nasal instillation group;
[0132] Group B: no-load group pVAX1 nasal instillation group;
[0133] Group C: negative control group-normal saline nasal cavity instillation group;
[0134] 3. Immunization scheme: Immunization once a week, continuous immunization for 3 weeks, a total of 3 times of immunization, and the immunization dose of each rat was 200 μg / time. 4. Sample collection: Collect saliva samples 1 day before immunization and 1, 2, 3, and 4 weeks after immunization.
[0135] 2% pilocarpine nitrate (0.1g / mL) was inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com