Inactivated yeast injection
A kind of injection, yeast technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Inoculate Saccharomyces cerevisiae (purchased from China Industrial Microorganism Culture Collection Management Center; Latin name: Saccharomycescerevisiae, storage number: CICC1012) into the medium (weigh 20g peptone, 20g glucose, 10g yeast powder, add water to 1000mL, sterilize at 121°C 20 min), cultured in a constant temperature oscillator at 37°C at a speed of 160r / min for 24 hours, then centrifuged at 3000 rpm for 5 minutes, removed the supernatant culture solution to retain the precipitate, added sterile saline to wash the precipitate, centrifuged for 5 minutes, and repeated washing for 3 After several times, add sterile saline, mix with the precipitate, and make a suspension. Take a certain amount of Saccharomyces cerevisiae physiological saline suspension, and count the bacteria on the THOMA bacterial counting plate, so that each 1ml suspension contains 10 9 A Saccharomyces cerevisiae cell. The prepared physiological saline suspension of Saccharomyces cerevisia...
Embodiment 2
[0023] Torulaspora delbrueckii (Torulaspora delbrueckii, purchased from China Industrial Microorganism Culture Collection Management Center; Latin name: Torulaspora delbrueckii, preservation number: CICC1004) was inoculated into the medium (weighed 20g peptone, 20g glucose, 10g of yeast powder, add water to 1000mL, sterilize at 121°C for 20min to prepare), incubate in a constant temperature shaker at 37°C at a speed of 160r / min for 24 hours, then centrifuge at 3000rpm for 5 minutes, remove the supernatant culture solution and retain the precipitate, add sterile Wash the precipitate with normal saline, centrifuge for 5 minutes, repeat the washing 3 times, add sterile normal saline, mix with the precipitate, and make a suspension. Take a certain amount of torula spores physiological saline suspension, and count the bacteria on a THOMA bacterial counting plate, so that each ml suspension contains 10 9A Torula spp. thallus. The prepared Torula delbuspora saline suspension was ina...
Embodiment 3
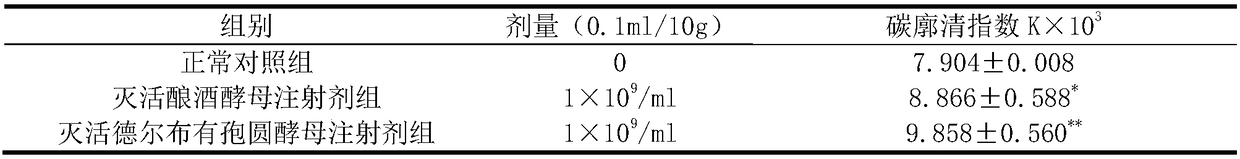
[0025] The effects of the inactivated Saccharomyces cerevisiae injection prepared in Example 1 and the inactivated Torula delbui injection prepared in Example 2 on the phagocytic function of normal mouse monocyte-macrophages were detected by the carbon particle clearance test method. The clean grade Kunming mice weighing 18-22 g were divided into normal control group, inactivated Saccharomyces cerevisiae injection group and inactivated Torula cerevisiae injection group. There were 10 mice in each group, half male and half male. The mice in the normal control group were injected with sterile normal saline in the tail vein, the mice in the inactivated S. cerevisiae injection group were injected with the inactivated S. Torula spp. suspension. The administration volume of each of the above groups was 0.1 mL / 10 g, and the tail vein was injected continuously for 5 days, once a day. 2 hours after the last administration, the mice were injected with 0.05 mL / 10 g of Indian ink into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com