Preparation and after-treatment method of p-tert-butylbenzoic acid
A technology of tert-butylbenzoic acid and p-tert-butyltoluene, which is applied in the field of its preparation and post-processing, can solve the problems of severe reaction heat release, high consumption of raw materials, and large amount of phthalic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
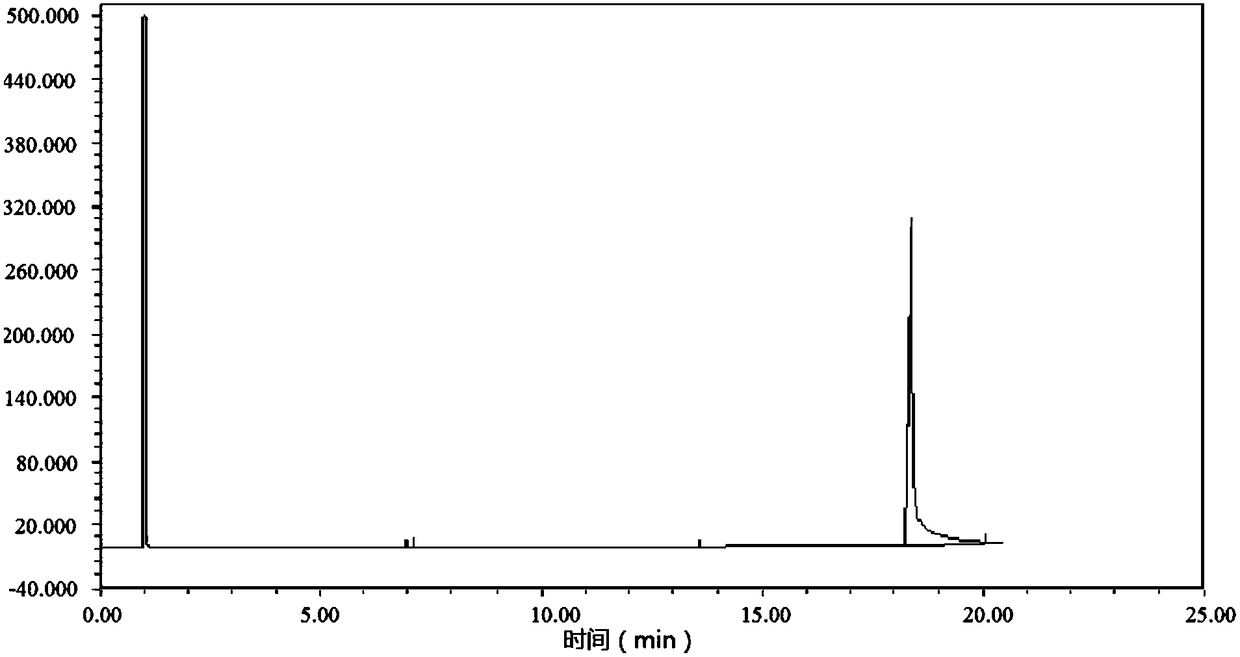
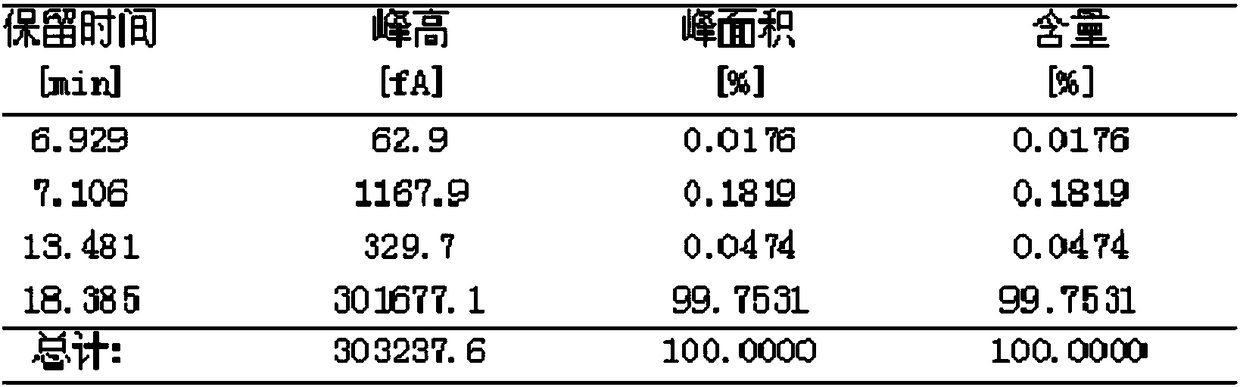
Image
Examples
preparation example Construction
[0037] The invention provides a kind of preparation and aftertreatment method of p-tert-butylbenzoic acid, described method comprises the following steps:
[0038] Step 1, utilizing toluene, isobutylene and concentrated sulfuric acid to prepare p-tert-butyltoluene, and then utilizing the obtained p-tert-butyltoluene to carry out oxidation reaction;
[0039] Step 2, carry out crystallization treatment after the oxidation reaction finishes, then centrifuge and wash to obtain the wet product of the crystallization mother liquor and the first batch of p-tert-butylbenzoic acid respectively, preferably the first batch of p-tert-butylbenzoic acid obtained The wet product is dried;
[0040] Step 3, adding aqueous sodium hydroxide solution to the crystallization mother liquor obtained in step 2, extracting to obtain the water phase, then adding concentrated sulfuric acid to the water phase, the residual p-tert-butylbenzoic acid in step 2 is precipitated, and filtered to obtain the seco...
Embodiment approach
[0045] According to a preferred embodiment of the present invention, step 1 includes the following sub-steps:
[0046] Step 1-1, mixing toluene and concentrated sulfuric acid, passing through isobutylene for alkylation reaction, removing acid after the reaction, adding alkaline water for extraction to obtain an aqueous phase and an organic phase, and performing rectification on the organic phase to obtain p-tert-butyltoluene;
[0047] Step 1-2, transfer the p-tert-butyltoluene obtained in step 1-1 into an oxidation kettle, add a catalyst, and feed air to carry out oxidation reaction.
[0048] According to a preferred embodiment of the present invention, in step 1-1, the alkylation reaction is carried out as follows: react at 6-14° C. for 8-15 hours.
[0049] In a further preferred embodiment, in step 1-1, the alkylation reaction is carried out as follows: react at 8-10° C. for 10.5-11.5 hours.
[0050] According to a preferred embodiment of the present invention, in step 1-1...
Embodiment 1
[0110] (1) 340g of toluene and 20g of concentrated sulfuric acid are added to the reactor, and 170g of isobutylene is introduced into the reaction kettle at 8° C. for alkylation for 11 hours. After the reaction, the acid is removed, and a 30% sodium carbonate aqueous solution is added to adjust the pH to neutral. Extract the water phase and the oil phase; then carry out rectification on the oil phase to obtain p-tert-butyltoluene.
[0111] Transfer the obtained p-tert-butyltoluene into an oxidation kettle, add 0.968g of cobalt acetate and 0.032g of manganese acetate into the kettle, raise the temperature to 125°C, and feed air at a rate of 2200mL / min to carry out the oxidation reaction. During the oxidation reaction process, control the produced water to 24-26g.
[0112] Among them, after the oxidation starts, the heat release in the oxidation process is stable. Although the reaction is stable, the temperature must be lowered, but the temperature reduction is easy to control, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com