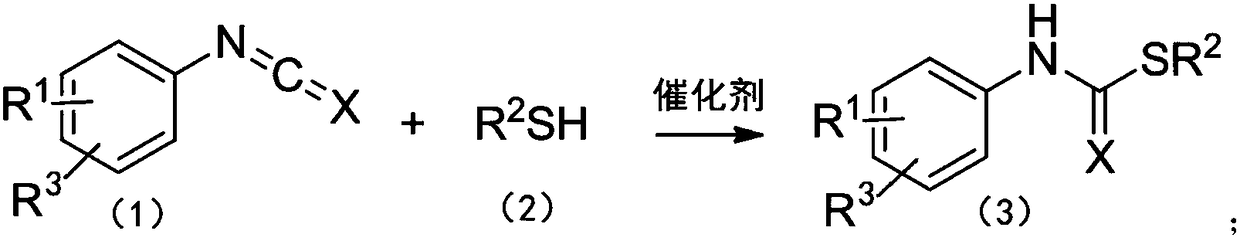
Method for catalyzing addition reaction of phenyl isocyanate or phenyl isothiocyanate with thiol
A technology of phenyl isothiocyanate and phenyl isocyanate, which is applied in the field of catalyzing the addition reaction of phenyl isocyanate or phenyl isothiocyanate and mercaptan, and can solve the problem of limited catalyst metal types and catalyst dosage Large size, poor substrate universality, etc., to achieve the effect of wide application range of substrates, mild reaction temperature and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
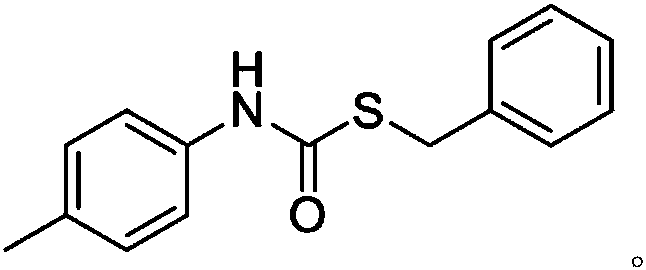
[0038] Example 1: 10mol% La[N(SiMe 3 ) 2 ] 3 Catalyzes the intermolecular addition reaction of phenyl isocyanate and benzyl mercaptan at 30 °C:
[0039] Anhydrous and oxygen-free, under the protection of argon, add 0.0620g (1.00×10 -4 Mole)La[N(SiMe 3 ) 2 ] 3 , then add 117.39μL (1.00×10 -3 mol) benzylthiol was stirred at room temperature for 15 min under an argon atmosphere, and 108.69 μL (1.00×10 -3 mol) phenyl isocyanate, stirred and reacted in a constant temperature bath at 30°C. After 24 hours, the protective argon gas was removed to quench the reaction, and the NMR yield was 83% according to the NMR analysis. The NMR data: 1 H NMR (400MHz, CDCl 3 ): δ7.39-7.13(m,9H,ArH),7.02(dd,J 1 =19.4,J 2 =12.0Hz, 2H, ArH, NH), 4.15(s, 2H, CH 2 )ppm. As can be seen from Comparative Example 1 and Example 1, La[N(SiMe 3 ) 2 ] 3 When used as a catalyst, its final yield is greatly improved compared with the yield without rare earth catalyst.
Embodiment 2
[0040] Example 2: 10mol% of Sm[N(SiMe 3 ) 2 ] 3 Catalyzes the intermolecular addition reaction of phenyl isocyanate and benzyl mercaptan at 30 °C:
[0041] Under anhydrous, oxygen-free and argon protection, add 0.0625g (1.00×10 -4 Mole)Sm[N(SiMe 3 ) 2 ] 3 , then add 117.39μL (1.00×10 -3 mol) benzylthiol was stirred at room temperature for 15 min under an argon atmosphere, and 108.69 μL (1.00×10 -3 mol) phenyl isocyanate, stirred and reacted in a constant temperature bath at 30°C. After 24 hours, the protective argon gas was removed and the reaction was quenched by air exposure. The NMR yield was 79% according to NMR analysis.
Embodiment 3
[0042] Example 3: 10mol% Yb[N(SiMe 3 ) 2 ] 3 Catalyzes the intermolecular addition reaction of phenyl isocyanate and benzyl mercaptan at 30 °C:
[0043] Anhydrous and oxygen-free, under the protection of argon, add 0.0655g (1.00×10 -4 Mole)Yb[N(SiMe 3 ) 2 ] 3 , then add 117.39μL (1.00×10 -3 mol) benzylthiol was stirred at room temperature for 15 min under an argon atmosphere, and 108.69 μL (1.00×10 -3 mol) phenyl isocyanate, stirred and reacted in a constant temperature bath at 30°C. After 24 hours, the protective argon gas was removed to vent the reaction to quench the reaction, and the NMR yield was 75% according to NMR analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com