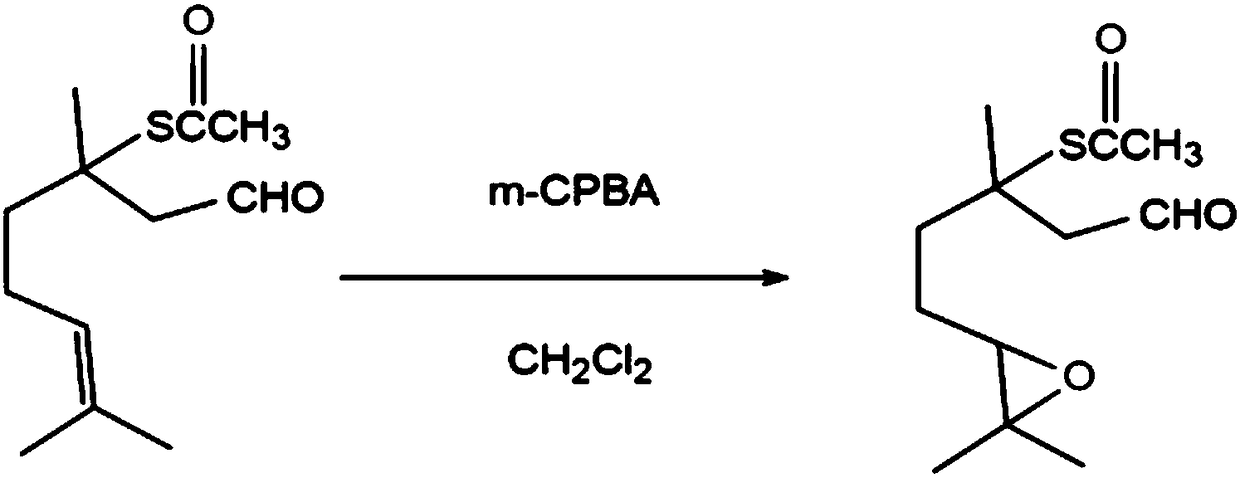
Preparation method of 3,7-dimethyl-3- acetylthio-6,7-epoxy-octanal
A technology of acetylmercapto and dimethyl, which is applied in the field of preparation of sulfur-containing terpenes, can solve the problems of many by-products, low yield, harsh reaction conditions, etc., and achieve the effects of low production cost, simple preparation process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal, comprising the following steps:
[0028] (1), at room temperature, 1.21g (98.90%, 5.25mmol) of 3,7-dimethyl-3-acetylmercapto-6-octenal and 1.07g (85.00%, 5.25mmol) of m-chlorobenzeneperformic acid ) was dissolved in 15.00 mL of dichloromethane, stirred and reacted for 4 hours, and the reaction was terminated, and the pH of the reacted solution was adjusted to neutral with a 5% aqueous sodium hydroxide solution by mass fraction to obtain a reaction solution;
[0029] (2), the neutral reaction solution gained in step (1) is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 0.98 g of crude product 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal, with a gas chromatography content of 91.36 %, yield 69.95%;
[0030] (3), the crude p...
Embodiment 2
[0034] A preparation method of 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal, comprising the following steps:
[0035](1) At room temperature, mix 1.41g (98.90%, 6.12mmoL) of 3,7-dimethyl-3-acetylmercapto-6-octenal and 1.49g (85.00%, 7.34mmol) of m-chlorobenzeneperformic acid ) was dissolved in 15.00 mL of dichloromethane, stirred and reacted for 4 hours, and the reaction was terminated, and the pH of the reacted solution was adjusted to neutral with a 5% aqueous sodium hydroxide solution by mass fraction to obtain a reaction solution;
[0036] (2), the neutral reaction solution gained in step (1) is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 1.20 g of 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal crude product, with a gas chromatography content of 93.56 %, yield 75.35%;
[0037] (3), the crude...
Embodiment 3
[0039] A preparation method of 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal, comprising the following steps:
[0040] (1), at room temperature, 1.51g (98.90%, 6.55mmol) of 3,7-dimethyl-3-acetylmercapto-6-octenal and 1.99g (85.00%, 9.83mmol) of m-chlorobenzeneperformic acid ) was dissolved in 20.00 mL of dichloromethane, stirred and reacted for 8 hours, and the reaction was terminated, and the pH of the reacted solution was adjusted to neutral with a 5% aqueous sodium hydroxide solution by mass fraction to obtain a reaction solution;
[0041] (2), the neutral reaction solution gained in step (1) is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 1.47g of crude product 3,7-dimethyl-3-acetylmercapto-6,7-epoxy-octanal, which is tested and obtained by gas phase The chromatographic content is 93.90%, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com