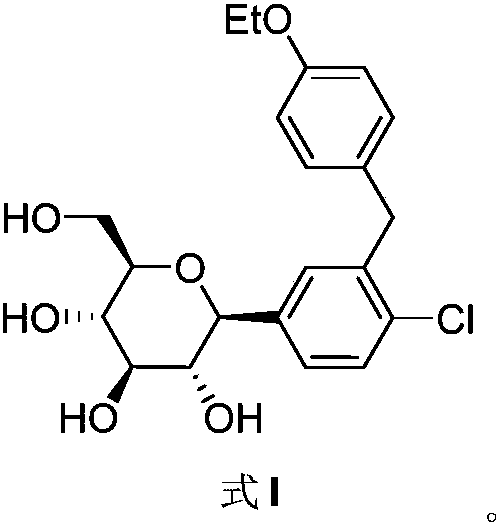
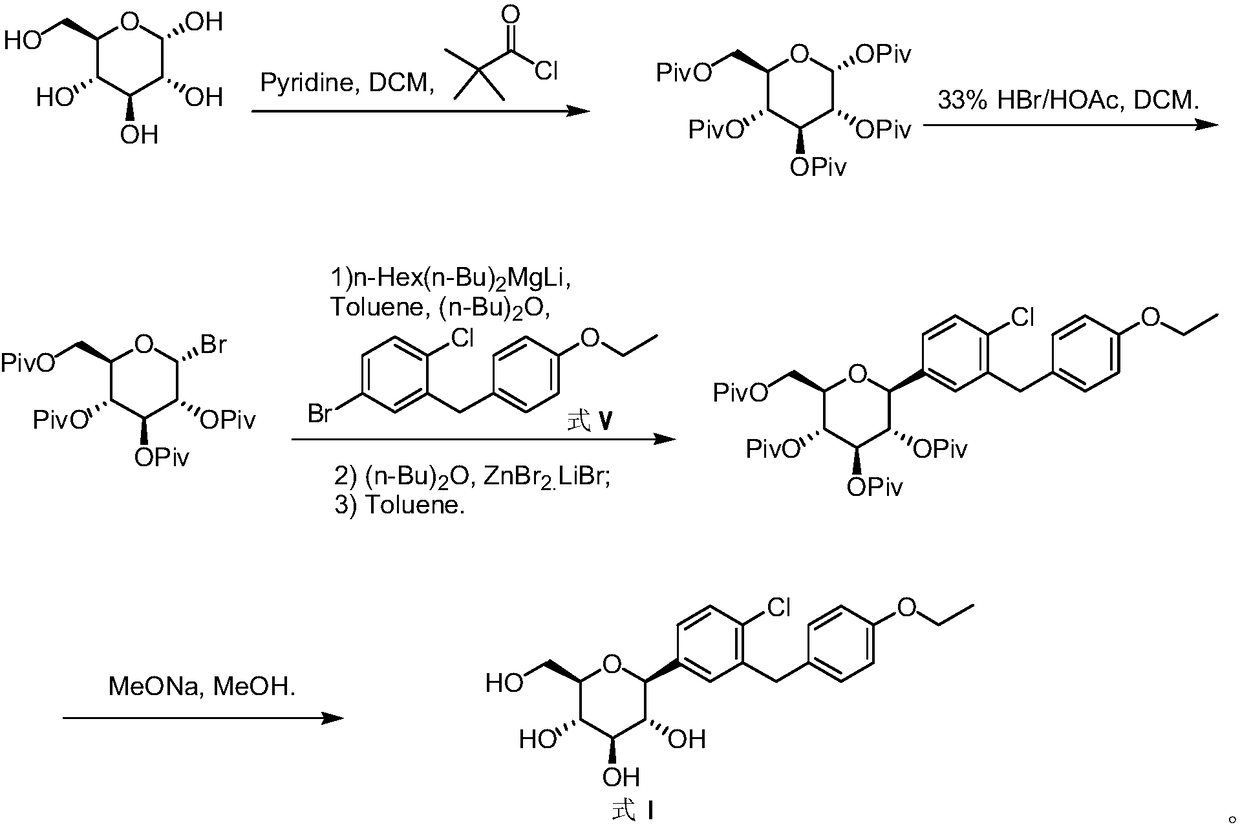
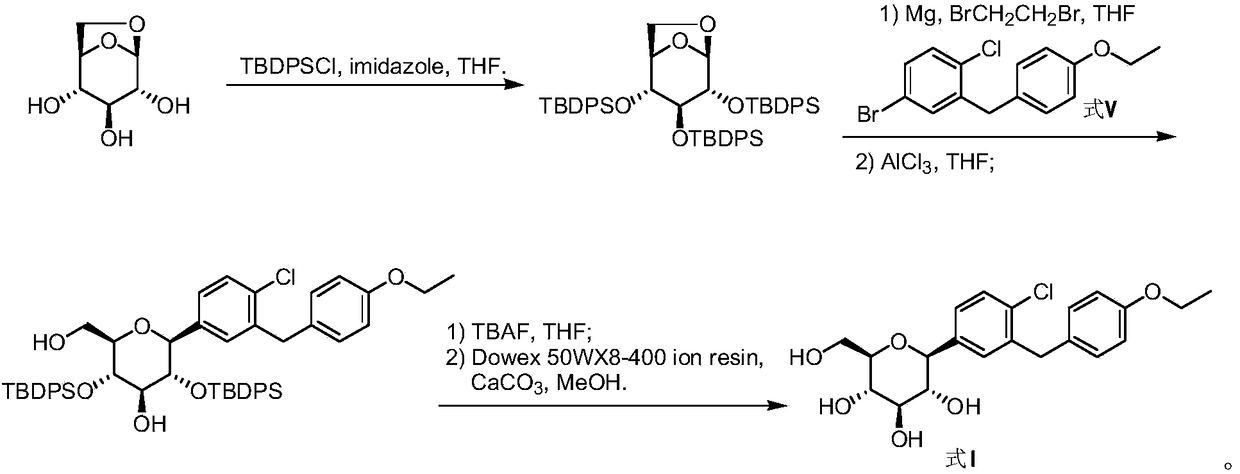
Method for preparing dapagliflozin
A compound and synthetic route technology, applied in the field of preparation of bulk drug dapagliflozin, can solve the problems of unfavorable base toxic impurities, long reaction steps, increased processing steps, etc., and achieves simple process operation, short synthetic route, and concise method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of 4-bromo-1-chloro-2[(4-ethoxy-phenyl)-diethoxy-methyl]benzene (formula III, R=Et)
[0025]
[0026] 500mL four-necked flask with magnetic stirring, thermometer and reflux condenser, successively added 5-bromo-2-chloro-4'-ethoxybenzophenone (formula II, 33.96g, 100mmol), triethyl orthoformate ( 22.2g, 150mmol), p-toluenesulfonic acid monohydrate (1.9g, 10mmol) and absolute ethanol (200mL), heated to 60+5°C under stirring for 6 hours, cooled to room temperature, added triethylamine (2.0g ) to adjust the pH to alkaline, and evaporate ethanol under reduced pressure. Add toluene (150mL) to dissolve the residue, wash the layers with water (2×60mL) twice, concentrate the organic layer under reduced pressure again, add heptane for beating, filter, and dry the obtained solid to give the white title compound (Formula III, R=Et , 32.5g, yield 78.6%).
Embodiment 2
[0027] Example 2: Preparation of 4-bromo-1-chloro-2[(4-ethoxy-phenyl)-dimethoxy-methyl]benzene (formula III, R=Me)
[0028]
[0029] 250mL four-necked flask with magnetic stirring, thermometer and reflux condenser, successively added 5-bromo-2-chloro-4'-ethoxybenzophenone (formula II, 16.98g, 50mmol), trimethyl orthoformate ( 15.92g, 150mmol), methanesulfonic acid (0.96g, 10mmol), anhydrous methanol (40mL) and toluene (120mL), heated to 60+5°C with stirring for 10 hours, cooled to room temperature, added triethylamine ( 1.5g) adjusted the pH to alkaline, washed the layers (2×100mL) twice, concentrated the organic layer under reduced pressure, added heptane for beating treatment, filtered, and dried the obtained solid to give the white title compound (Formula III, R=Me, 15.9 g, yield 82.4%).
Embodiment 3
[0030]Example three: (4-ethoxy-phenyl)-[3-(2,3,4,5-tetrahydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yl)-phenyl] - Preparation of ketone (formula IV)
[0031]
[0032] 250mL four-neck bottle rack with magnetic stirring, thermometer, constant pressure dropping funnel and reflux condenser, under the protection of positive nitrogen pressure, add 4-bromo-1-chloro-2[(4-ethoxy-phenyl)-dimethyl Oxy-methyl]benzene (formula III, R=Me, 3.86g, 10mmol), anhydrous THF (20mL) and magnesium chips (25.2, 100mmol), heated to 50°C under stirring, and continued stirring until the reaction was stabilized , start dropwise adding 4-bromo-1-chloro-2[(4-ethoxy-phenyl)-dimethoxy-methyl]benzene (formula III, R=Me, 34.7 g, 90mmol) dissolved in anhydrous THF (160mL) solution, exotherm is obvious, control the internal temperature during the dropwise addition between 40-55°C, after the dropwise addition is completed, keep warm at 40-55°C for 1 hour to obtain a gray grid Reagent solution, under nitrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com