Canagliflozin impurity compound and preparation method thereof
A compound and oxidant technology, applied in organic chemistry, measuring devices, instruments, etc., can solve problems such as low product purity and lack of single impurity control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
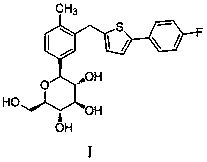
[0038] Canagliflozin (Formula I) can be prepared, for example, according to the method reported in patent WO2005012326 (same family patent CN1829729), the entirety of which is incorporated into the present invention for reference. In addition, it can also be purchased through commercial channels.
[0039] The instruments used for mass spectrometry were Agilent 1200 high performance liquid chromatography system and Agilent G6410A tandem triple quadrupole mass spectrometer. The ion source was electrospray ion source in positive ion mode. Split the HPLC eluate, allowing approximately 1 μg / ml into the ion source of the mass spectrometer.
[0040] The instrument used for nuclear magnetic resonance analysis is Bruker Avance 600 nuclear magnetic resonance spectrometer, and the deuterated solvent is DMSO-d6. TMS was used as a proton resonance (δ1H 0.00) and solvent internal reference, and DMSO-d6 as carbon resonance internal standard (δ13C 39.10-40.10).
[0041] The following abbrevi...
Embodiment 1
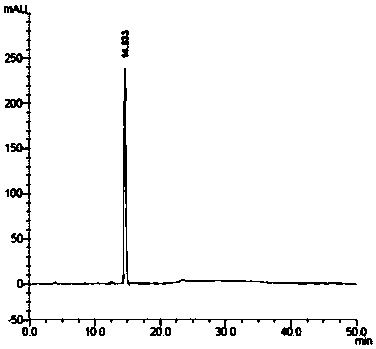
[0043] Embodiment 1: the HPLC purity analysis method of canagliflozin impurity IV
[0044] Take about 12.5mg of this product, weigh it accurately, put it in a 25ml measuring bottle, add acetonitrile to dissolve and dilute to the mark, shake well, and use it as the test solution; test according to the high performance liquid chromatography (General Rule 0512 of the Chinese Pharmacopoeia 2015 Edition). Butylsilane bonded silica gel is used as filler (C4, 4.6mm×250mm, 5μm); water is used as mobile phase A, acetonitrile is used as mobile phase B, and elution is carried out according to the gradient elution table; the flow rate is 1.0 per minute ml; the detection wavelength is 210nm; the column temperature is 25°C; precisely measure 20μl of the test solution and inject it into the liquid chromatograph, and the collection time is 60min. Should meet the requirements. In the chromatogram of the test solution, except for the solvent peak, calculated by the area normalization method, t...
Embodiment 2
[0046] Embodiment 2: the preparation of formula II compound
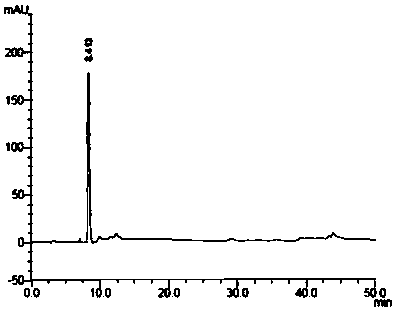
[0047] Dissolve canagliflozin (30g, 67.5mmol) in 300ml absolute ethanol, slowly add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (30.6 g, 134.8mmol) in absolute ethanol solution (240ml), react at room temperature for about 24 hours, TLC detects that the reaction is complete, stop the reaction. Under cooling in an ice bath, slowly pour the reaction solution into a mixture of dichloromethane and water, separate the liquids, extract the water phase twice with dichloromethane, combine the dichloromethane layers, dry over anhydrous magnesium sulfate, filter, and reduce Concentrate under pressure to dryness to obtain crude product.
[0048] The crude product was purified by column chromatography using a mixed system of methanol and dichloromethane as the eluent to obtain [2-methyl-5-(β-D-glucopyranosyl)phenyl][5-(4-fluoro Phenyl)-2-thienyl]methanone (compound of formula II), HPLC: 99.3%.
[0049] The M+H+ peak in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com