Alkyl pyridine imine iron-based catalyst and preparation method and application thereof
A kind of alkyl pyridine imide iron series, pyridine imide iron technology, applied in the application of isoprene polymerization, catalyst preparation, alkyl pyridine imide iron series catalyst field, can solve the problem of low activity, molecular weight distribution Wide, unclear active center and other problems, to achieve the effect of small temperature dependence, good industrial value, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
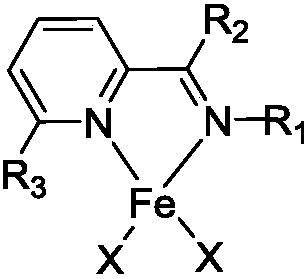
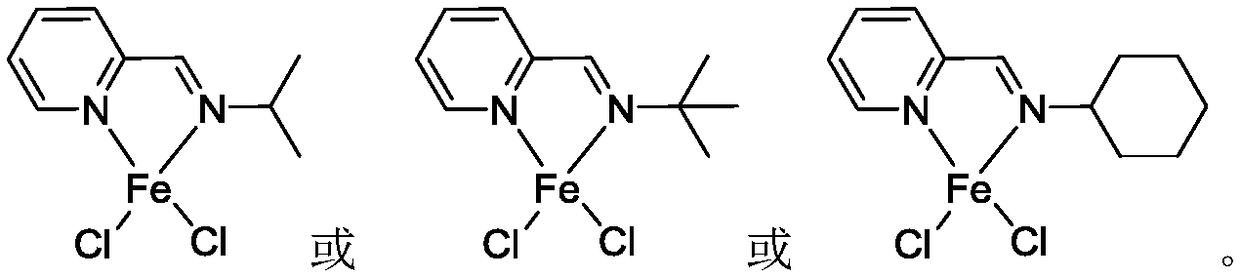
[0044]The present embodiment prepares the pyridine imine iron complex shown in formula (1):
[0045] The 25mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl 2 And isopropyl substituted pyridinimine ligand, stirred at room temperature for 24h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain a red solid.
[0046]
[0047] Mass Spectrometry: C 9 h 12 ClFeN 2 [M-Cl] + : Theoretical value: 239.0033; measured value: 239.0033.
[0048] Elemental Analysis: C 9 h 12 Cl 2 FeN 2 : Theoretical value: C, 39.32%; H, 4.40%; N, 10.19%; Measured value: C, 39.39%; H, 4.45%; N, 10.17%.
[0049] Magnetic susceptibility: (500MHz, CD 2 Cl 2 ):μ eff =4.92μ B (5.0 mg / mL).
[0050] H NMR spectrum: 1 H NMR (500MHz, CD 2 Cl 2 ,25℃,δ):88.2(Δ ν1 / 2 =356Hz), 66.8(Δ ν1 / 2 ...
Embodiment 2
[0052] The preparation process of the pyridinium iron complex shown in formula (2) prepared in this embodiment is as follows:
[0053] The 25mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl 2 And tert-butyl substituted pyridine imine ligand, stirred at room temperature for 24h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain a dark red solid.
[0054]
[0055] Mass Spectrometry: C 10 h 14 ClFeN 2 [M-Cl] + : Theoretical value: 253.0189; measured value: 253.0188.
[0056] Elemental Analysis: C 10 h 14 Cl 2 FeN 2 : Theoretical value: C, 41.56%; H, 4.88%; N, 9.69%; Measured value: C, 41.48%; H, 4.84%; N, 9.72%.
[0057] Magnetic susceptibility: (500MHz, CD 2 Cl 2 ):μ eff =5.21μ B (2.5 mg / mL).
[0058] H NMR spectrum: 1 H NMR (500MHz, CD 2 Cl 2 ,...
Embodiment 3
[0060] The pyridine imine iron complex shown in the formula (3) prepared in this embodiment, the preparation process is as follows:
[0061] The 10mL Schlenk reaction tube was pumped and baked three times, and 5mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl2 and cyclohexyl-substituted pyridinimine ligand were sequentially added into the glove box, and stirred at room temperature for 48h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain a reddish-purple solid.
[0062]
[0063] Mass Spectrometry: C 12 h 16 ClFeN 2 [M-Cl] + : Theoretical value: 279.0346; Measured value: 279.0347.
[0064] Elemental Analysis: C 12 h 16 Cl 2 FeN 2 : Theoretical value: C, 45.75%; H, 5.12%; N, 8.89%; Measured value: C, 45.84%; H, 5.16%; N, 8.83%.
[0065] Magnetic susceptibility: (500MHz, CD 2 Cl 2 ):μ eff =5.08μ B (2.5 mg / mL).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com