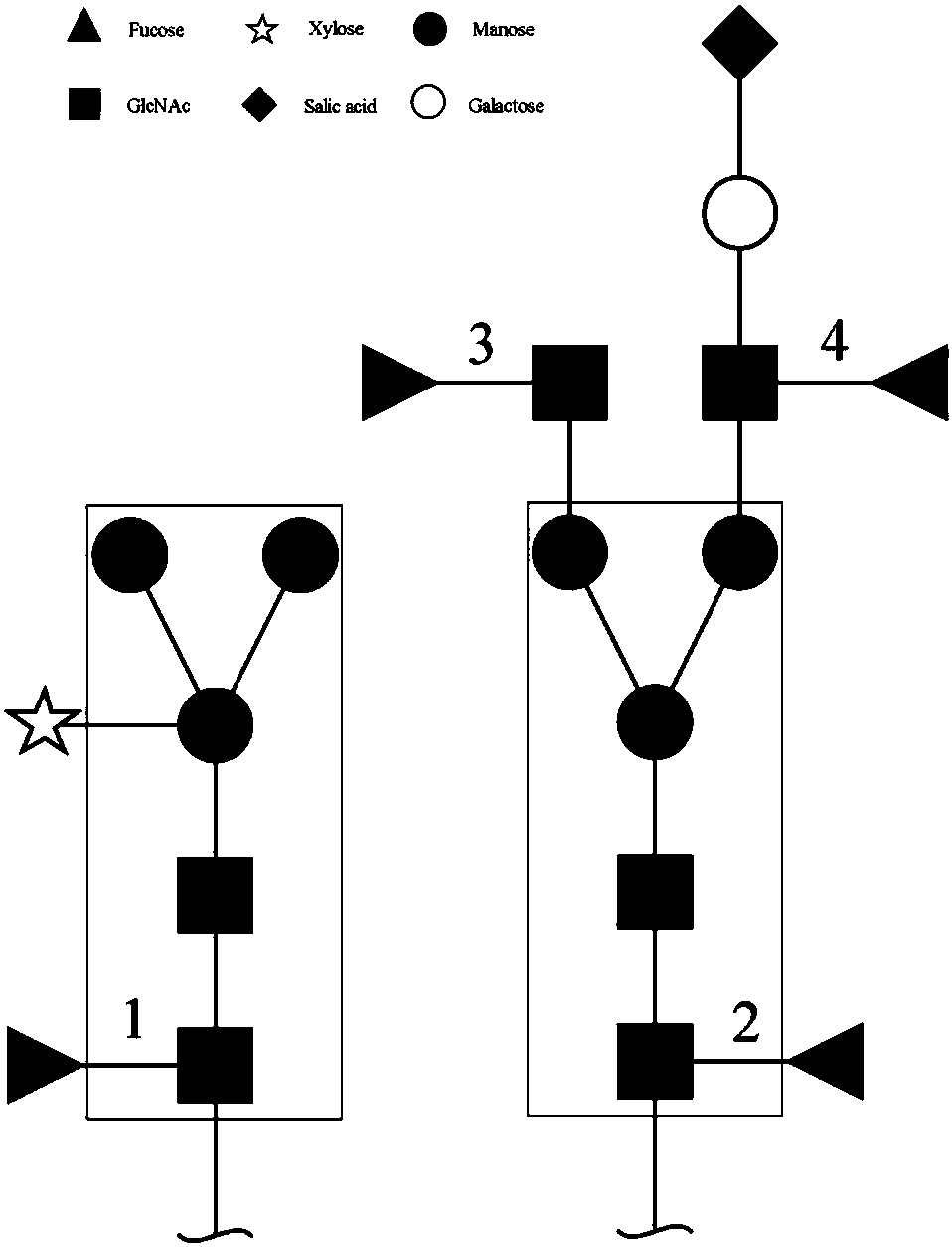
Core fucosidase as well as preparation and application thereof
A technology of fucosidase and fucose, which is applied in the field of glycobiology to achieve the effect of simple operation and high enzyme cutting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] Preparation of reaction substrate
[0068] Use three types of substrates to carry out the proof of this enzymatic activity among the present invention,
[0069] 1. The chromogenic substrate pNP-α-L-Fuc was purchased from carbosynth, UK;
[0070] 2.3-F, Lewis X oligosaccharides were purchased from carbosynth company in the UK;
[0071] 3. Glycoprotein horseradish peroxidase (HRP) was purchased from sigma;
[0072] The substrate pNP-α-L-Fuc and various oligosaccharides were reconstituted with ultrapure water as a preservation solution, and the glycoprotein HRP was prepared at a concentration of 10mg / ml for preservation;
[0073] The chromogenic substrate pNP-α-L-Fuc and oligosaccharides do not need to be heat-treated, and HRP is prepared with 25mM sodium dihydrogen phosphate solution (pH=4.6) at a concentration of about 0.5mg / ml, and DTT is added to make the final concentration of 10mM, heating at 93°C for 10min, thermal denaturation;
Embodiment 1
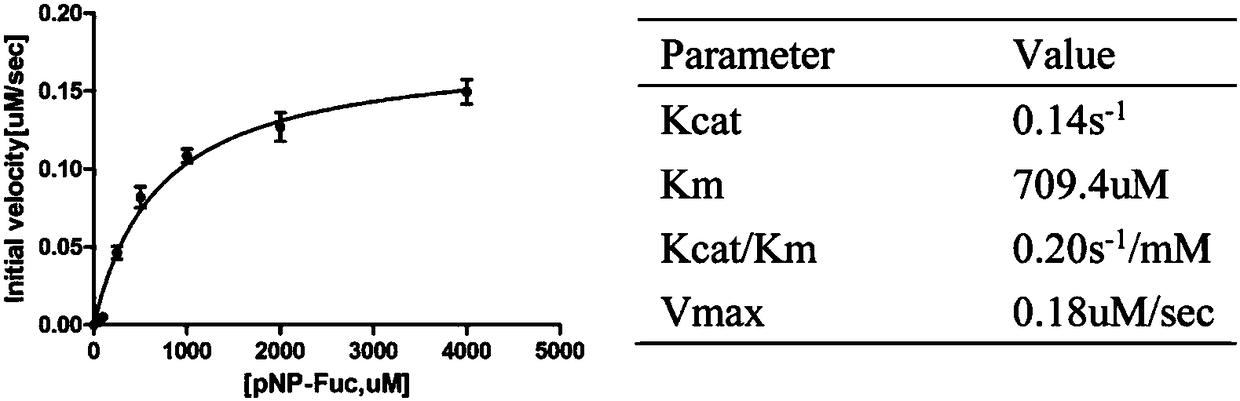
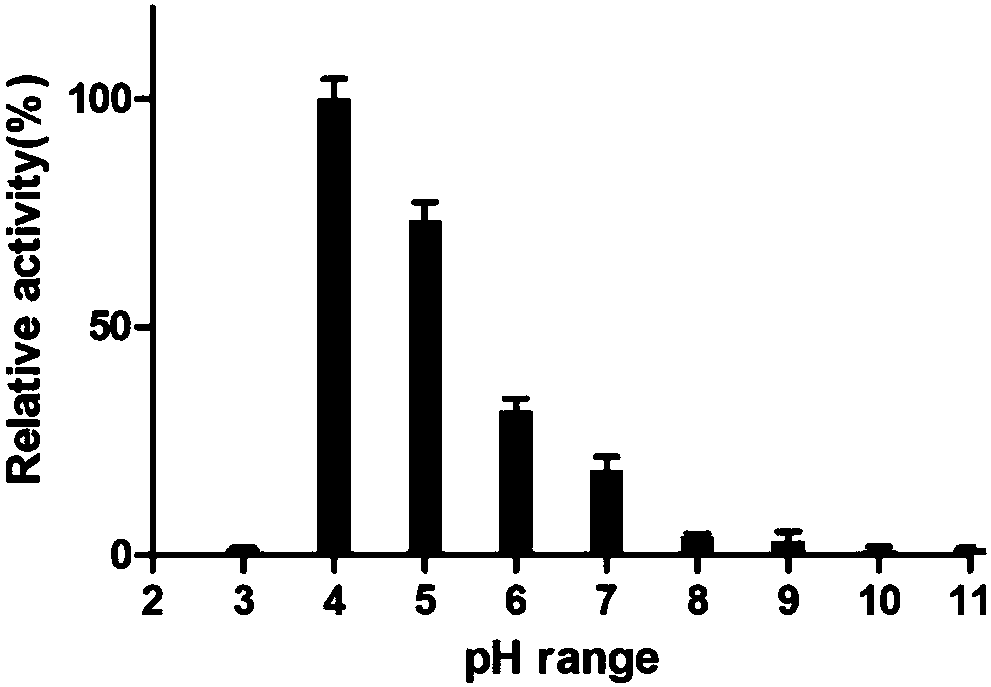
[0088] Example 1: Fucosidase Activity Identification and Enzymatic Properties Experiment
[0089] 1. Identification of fucosidase activity
[0090] Preparation of the reaction substrate: the substrate pNP-α-L-Fuc lyophilized powder (purchased from carbosynth company) was rethawed with distilled water at a concentration of 10 mM, and stored as a preservation solution.
[0091] Enzyme digestion reaction buffer: 25mM sodium dihydrogen phosphate (PH4.5-5.0)
[0092] Enzyme digestion system: Substrate pNP-α-L-Fuc preservation solution 10ul, enzyme 1ul, add phosphate buffer to make up to 60ul volume. In the table below, No. 1 is the experimental group, and No. 2 is the control group.
[0093] Table 1. Configuration of reaction system
[0094]
[0095] Reaction conditions: 37°C, 1h, immediately after the reaction, add 1M Na 2 CO 3 90ul, stop reaction, and carry out OD=405nm measurement with microplate reader;
[0096] Based on the above results, it was judged whether the en...
Embodiment 2
[0111] Example 2: Enzyme digestion experiment of oligosaccharides
[0112] Substrate configuration: the oligosaccharide 3-Fucosyllactose (3FL), Lewis x trisaccharide (Lex), was configured to a concentration of 10mM with ultrapure water;
[0113] Reaction system: phosphate buffer 8ul, oligosaccharide preservation solution 1ul, enzyme 1ul, a total of 10ul reaction system, 37 ° C for 1 hour;
[0114] Detection of enzyme digestion reaction: two methods are used in this embodiment to detect the products after enzyme digestion of oligosaccharides:
[0115] Method 1: detect according to the L-fucose detection kit (megazyme, Ireland), the specific method can be found in the kit manual, the kit uses fucose dehydrogenase to oxidize fucose, and NADP+ to generate NADPH NADPH can be measured by the absorbance value at 340nm and quantified according to the calibration curve, so as to quantify the fucose produced by enzymatic cleavage of oligosaccharides;
[0116] Method 2: Detect accordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com