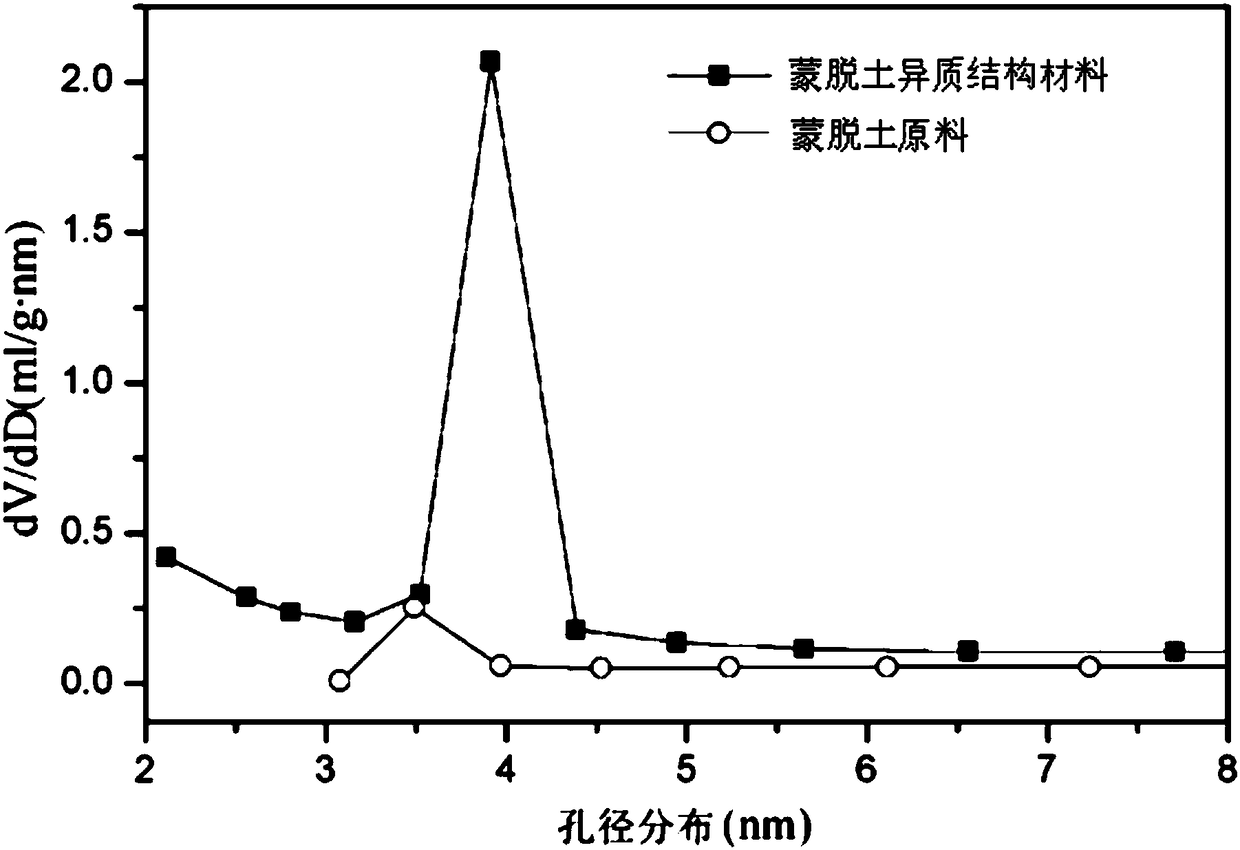
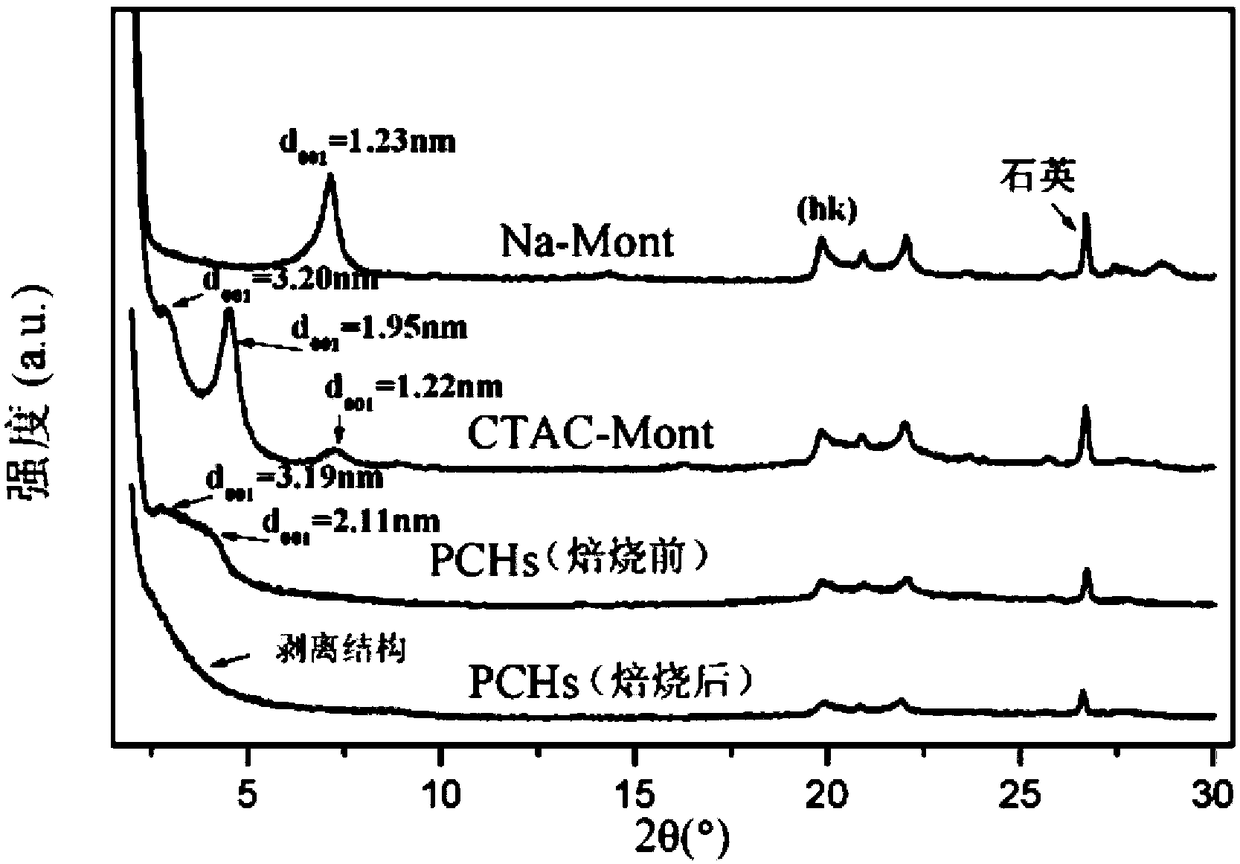
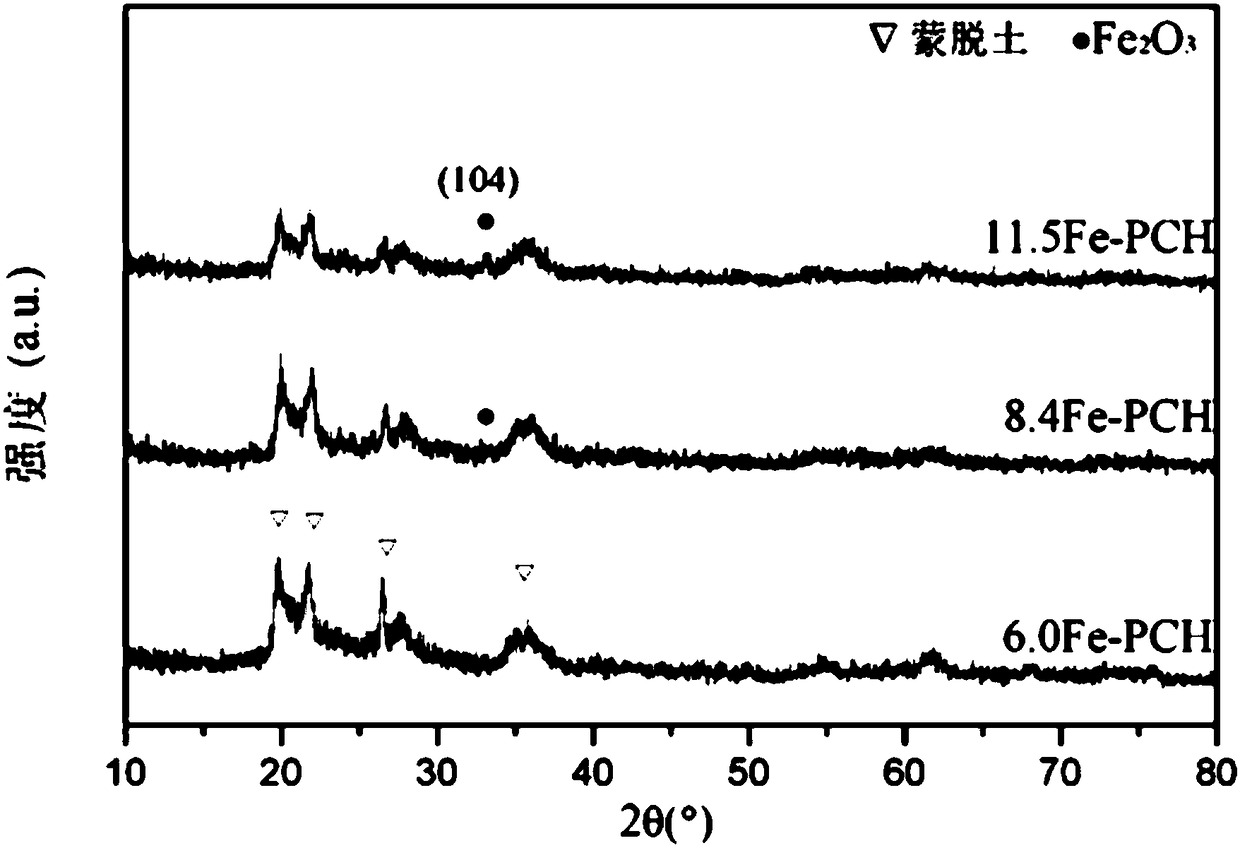
Fe-PCH catalyst as well as preparation method and application thereof
A catalyst, fe-pch technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve the problems of easy leakage of NH3, high cost of vanadium-titanium catalysts, secondary environmental pollution, etc. The effect of recycling treatment, high thermal stability and high catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of organically modified montmorillonite material: Add 1g of montmorillonite raw material to 100ml of NaCl solution with a concentration of 1mol / L, stir vigorously at 60°C for 12h, and then use a high-speed centrifuge for centrifugation (9000RPM, time 5 min), and the obtained sample was dried in a drying oven at 110° C. for 6 h to obtain sodium montmorillonite. Prepare 50ml of cetyltrimethylammonium chloride aqueous solution with a concentration of 0.1mol / L, mix it with 2.5g of the obtained sodium montmorillonite, stir vigorously in a water bath at 60°C for 24h using a magnetic stirrer, and then wash with deionized water 3 to 4 times until the pH value of the washing liquid is 7, the sample is separated by centrifugation as described above, and dried at room temperature for 24 hours to obtain an organically modified montmorillonite material.
[0028] (2) Preparation of montmorillonite heterostructure material: Dry the required utensils in a 110° C. drying...
Embodiment 2
[0031](1) Preparation of organically modified montmorillonite material: Add 1g of montmorillonite raw material to 100ml of NaCl solution with a concentration of 1mol / L, stir vigorously at 60°C for 12h, and then use a high-speed centrifuge for centrifugation (9000RPM, time 5 min), and the obtained sample was dried in a drying oven at 110° C. for 6 h to obtain sodium montmorillonite. Prepare 50ml of cetyltrimethylammonium chloride aqueous solution with a concentration of 0.1mol / L, mix it with 2.5g of the obtained sodium montmorillonite, stir vigorously in a water bath at 60°C for 24h using a magnetic stirrer, and then wash with deionized water 3 to 4 times until the pH value of the washing liquid is 7, the sample is separated by centrifugation as described above, and dried at room temperature for 24 hours to obtain an organically modified montmorillonite material.
[0032] (2) Preparation of montmorillonite heterostructure material: Dry the required utensils in a 110° C. drying ...
Embodiment 3
[0037] (1) Preparation of organically modified montmorillonite material: Add 1g of montmorillonite raw material to 100ml of NaCl solution with a concentration of 1mol / L, stir vigorously at 60°C for 12h, and then use a high-speed centrifuge for centrifugation (9000RPM, time 5 min), and the obtained sample was dried in a drying oven at 110° C. for 6 h to obtain sodium montmorillonite. Prepare 50ml of cetyltrimethylammonium chloride aqueous solution with a concentration of 0.1mol / L, mix it with 2.5g of the obtained sodium montmorillonite, stir vigorously in a water bath at 60°C for 24h using a magnetic stirrer, and then wash with deionized water 3 to 4 times until the pH value of the washing liquid is 7, the sample is separated by centrifugation as described above, and dried at room temperature for 24 hours to obtain an organically modified montmorillonite material.
[0038] (2) Preparation of montmorillonite heterostructure material: Dry the required utensils in a 110° C. drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com