Hexavanadate-L-alanine methyl ester derivative and preparation method and application thereof
A technology of alanine methyl ester and hexavanadate succinic acid, which is applied in the directions of organic chemistry methods, chemical instruments and methods, 5/15 groups of organic compounds without C-metal bonds, etc., can solve the problem of insufficient medicinal activity. Research, the development and application of polyvanadate drugs are slow and other issues, to achieve significant research value and application prospects, significant inhibitory activity, and the effect of yield on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
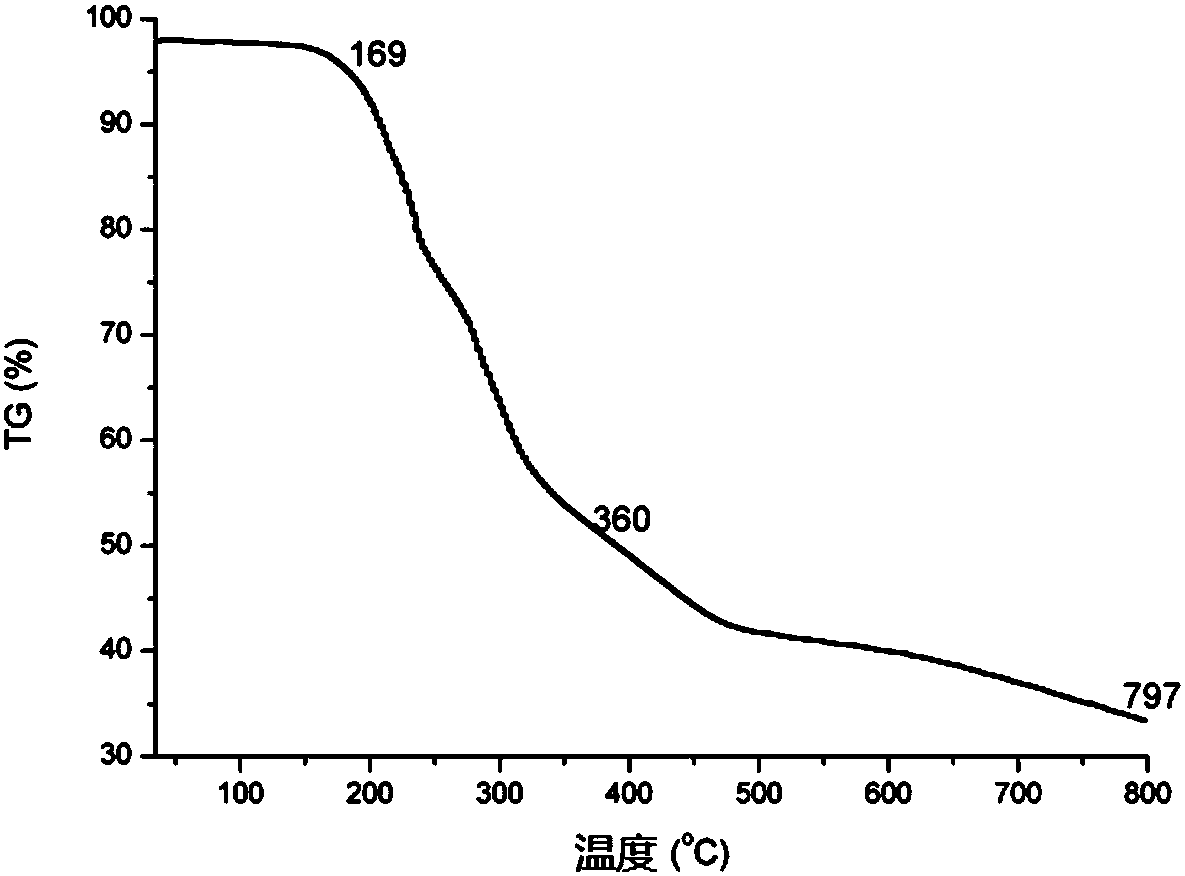
Image
Examples
Embodiment 1 6
[0025] The synthetic method of embodiment 1 hexavanadate-L-alanine methyl ester derivatives
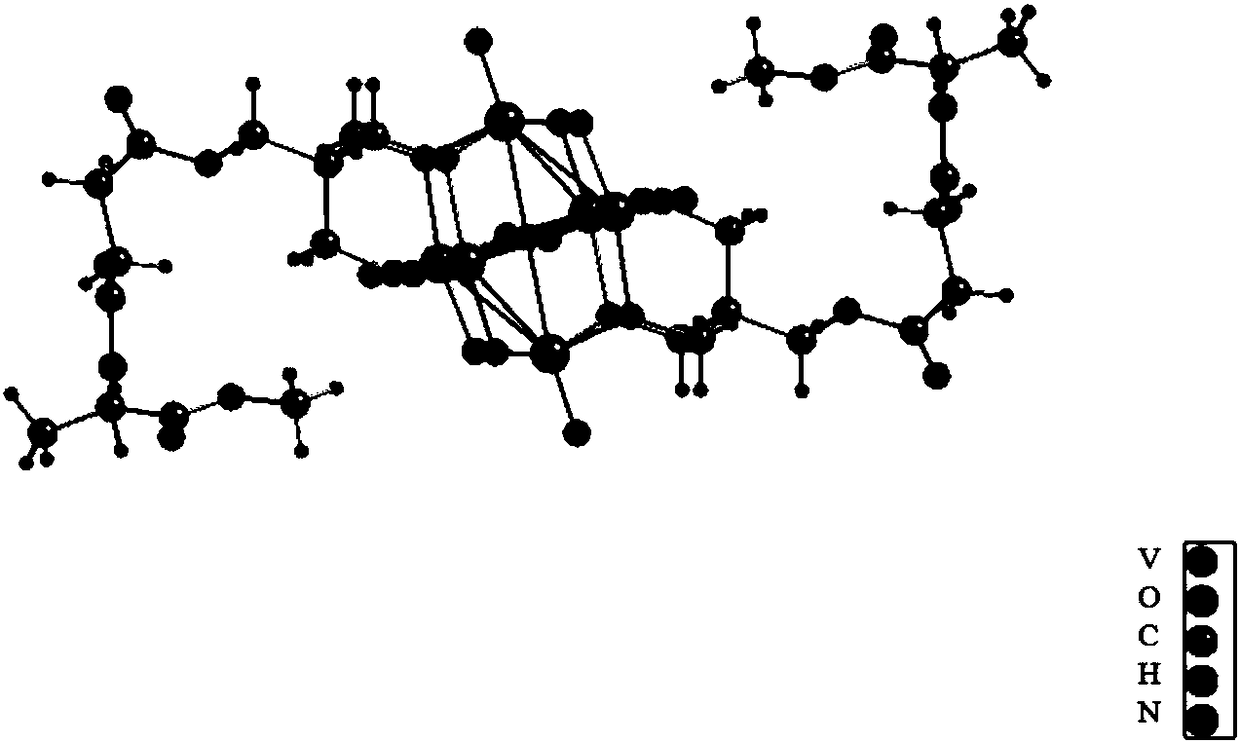
[0026] The anion structure of hexavanadate-L-alanine methyl ester derivative is as follows figure 1 As shown, it is Linqivist-type hexavanadate, in which all the vanadium is +5, and the oxygen atom on the hexavanadate is replaced by the hydroxyl oxygen of two trimethylol compounds, and each trimethylol The three hydroxyl oxygens on the compound replace the three bridging oxygen atoms arranged in a planar triangle on the hexavanadate, and the two trimethylol compounds are relatively distributed on the hexavanadate skeleton. The compounds are covalently linked.
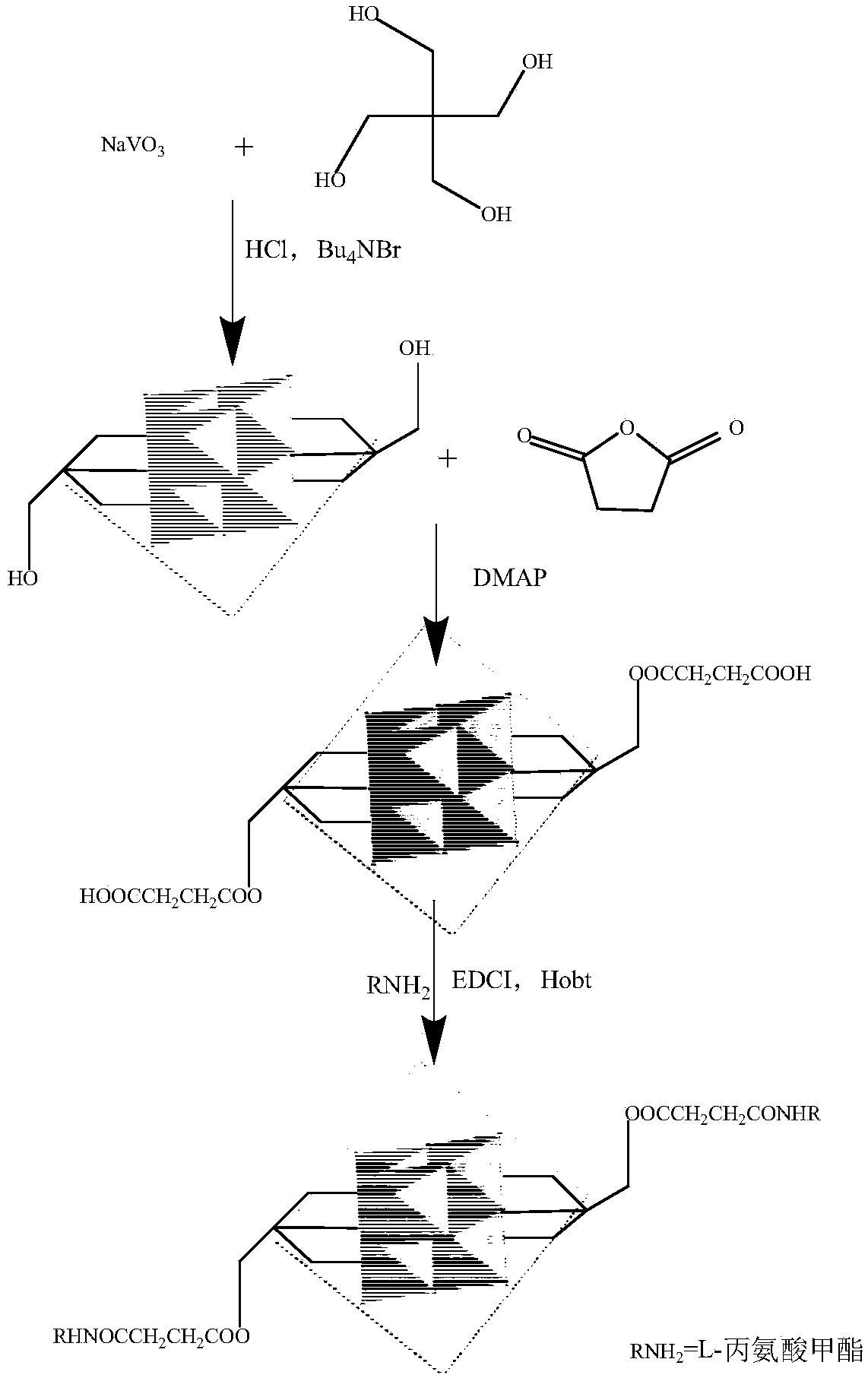
[0027] The synthesis of hexavanadate-L-alanine methyl ester derivatives is carried out in three steps, and the schematic diagram of the synthesis process is as follows: figure 2 shown.
[0028] (1)[Bu 4 N] 2 [V 6 o 13 {(OCH 2 ) 3 CCH 2 OH} 2 ] Synthesis of Pentaerythritol Hexavanadate Derivatives
[0029] Weigh 5g ...
Embodiment 2
[0035] Embodiment 2 antitumor activity evaluation
[0036] (1) In vitro antitumor activity evaluation of compounds
[0037] Tested tumor cells: human liver cancer cell HepG2, human rhabdomyoma cell RD, human cervical cancer cell Hela, human laryngeal cancer cell Hep-2, human breast cancer cell MCF-7.
[0038] Cell culture: GIBCO DMEM medium, 10% fetal bovine serum and 0.01% L-glutamine were prepared as culture medium. Cultured cell lines were placed at 37°C, 5% CO 2 The cells were routinely cultured and subcultured under saturated humidity, and the cells in the logarithmic growth phase were used in the experiments.
[0039] In vitro anti-tumor activity evaluation (MTT method): the above tumor cells were respectively plated in 96-well plates, and at 37°C, 5% CO 2 After the incubator was cultured to cover the monolayer, the cell culture medium was discarded, and the cell maintenance solution (containing serum 2% and 0.002% L-glutamine) containing different concentrations of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com