A kind of synthetic technique of tridemorpholine
A synthesis process, the technology of tridecamorpholine, applied in organic chemistry and other directions, can solve the problem of many by-products, and achieve the effects of reducing corrosion intensity, mild reaction conditions, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 500g tridecyl alcohol, 500g tridecane are added in the reactor;
[0066] Add 10g of catalysts (mixed by methyl copper, binuclear methyl nickel, dimethyl zinc and trimethyl aluminum in a weight ratio of 1:1:1:1) in the reactor, mix under stirring, Introduce hydrogen gas at a rate of 0.1L, and raise the temperature of the reactor to 230°C in about 2 hours;
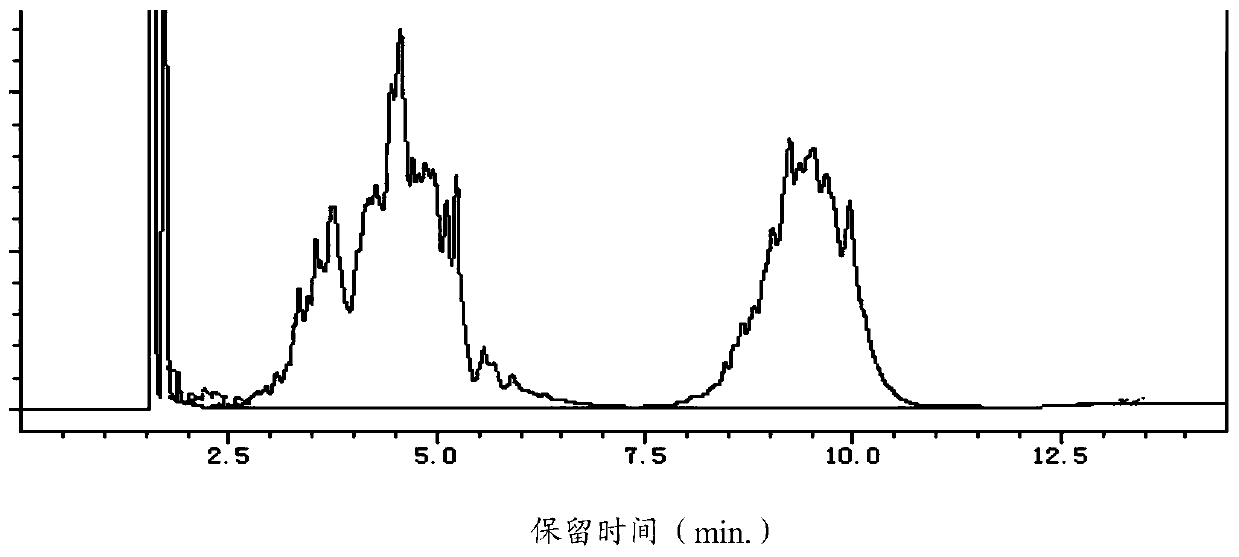
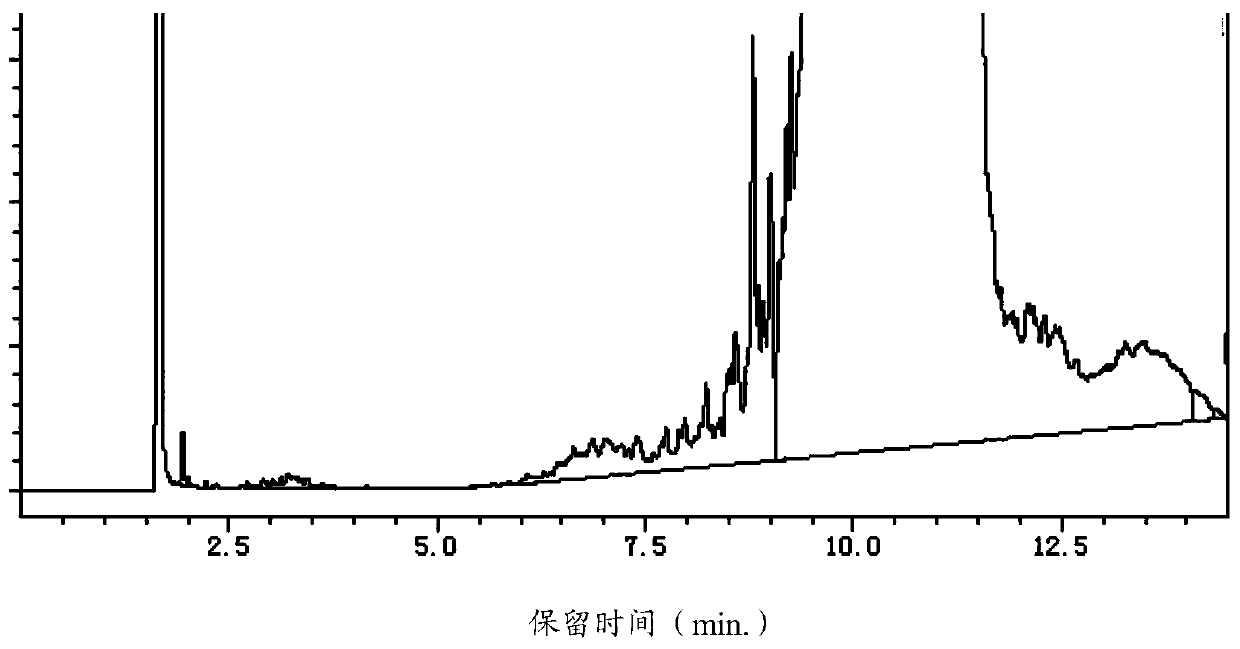
[0067] When the temperature of the reactor rises to 230°C, start to slowly add 400g of 2,6-dimethylmorpholine dropwise, maintain the pressure in the reactor at 0.3Mpa, and the temperature at 230-240°C until the end of the reaction. Or intermittently water and unreacted 2,6-dimethylmorpholine are taken out from the top of the tower; during the reaction, the reaction sample is detected by GC, such as figure 1 As shown, peaks appear at retention times (min.) of 1.716, 1.838, 1.891, 2.014, 2.273, 2.357, 2.447, 2.530, 4.563, 9.237, 13.301, 13.379, wherein 1.716, 4.563, 9.237 correspond to 2,6 - Dimethylmorpholine (8.299%...
Embodiment 2
[0071] 500g tridecyl alcohol, 400g tridecane are added in the reactor;
[0072] Add 9g of the fresh catalyst used in Example 1 and 2g of the catalyst recovered from Example 1 in the reactor, mix under stirring, feed hydrogen, and heat up the reactor;
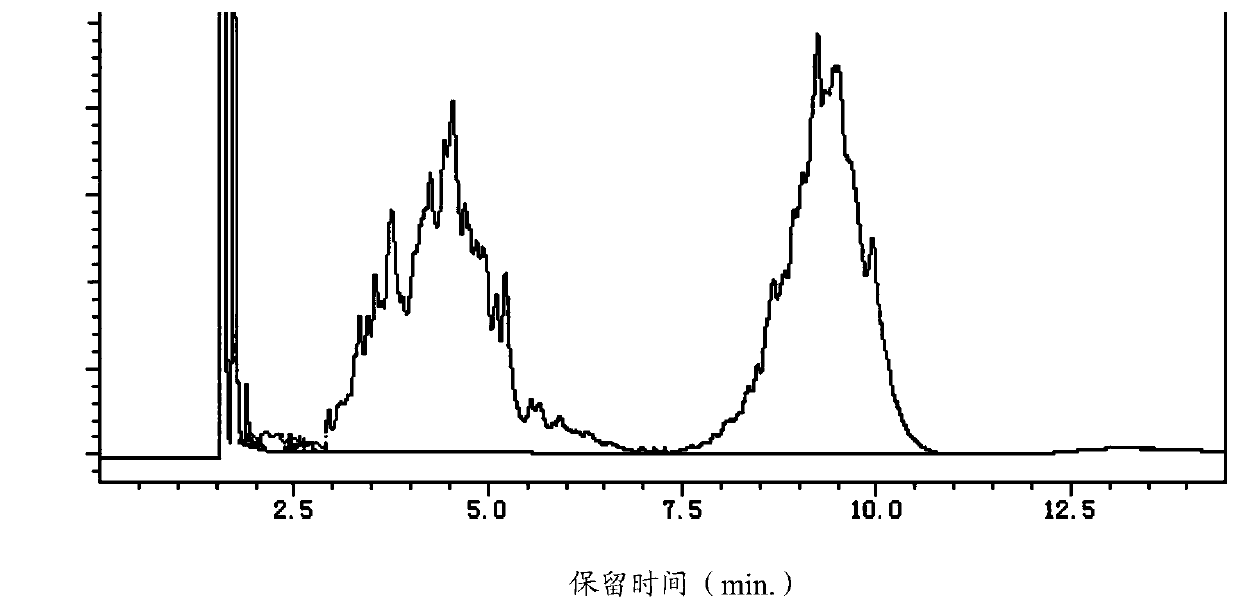
[0073] When the temperature of the reaction kettle rose to 225°C, slowly drop 400g of 2,6-dimethylmorpholine and 50g of 2,6-dimethylmorpholine recovered and purified by distillation in Example 1, and keep the reaction kettle The pressure is 0.35Mpa, and the temperature is 230-245°C until the end of the reaction; during the reaction, the reaction sample is detected by GC, such as image 3 As shown, peaks appear at the retention times (min.) of 1.717, 1.840, 1.891, 2.013, 2.061, 2.343, 2.444, 2.576, 2.767, 4.545, 6.983, 7.147, 7.261, 9.238, 13.272, wherein 1.717, 4.545, 9.238 corresponds to 2,6-dimethylmorpholine (7.414%), tridecane+tridecyl alcohol (45.986%), and tridecylmorpholine (45.947%), respectively.
[0074] After the dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com