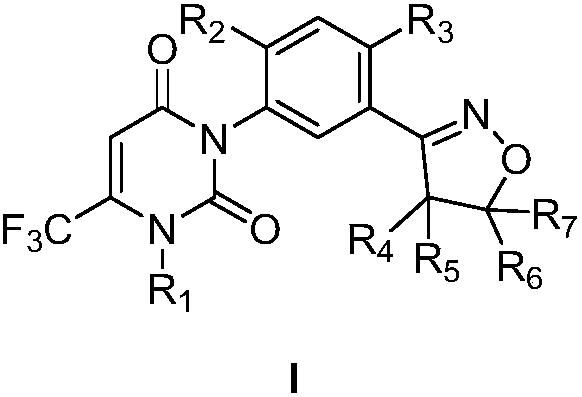
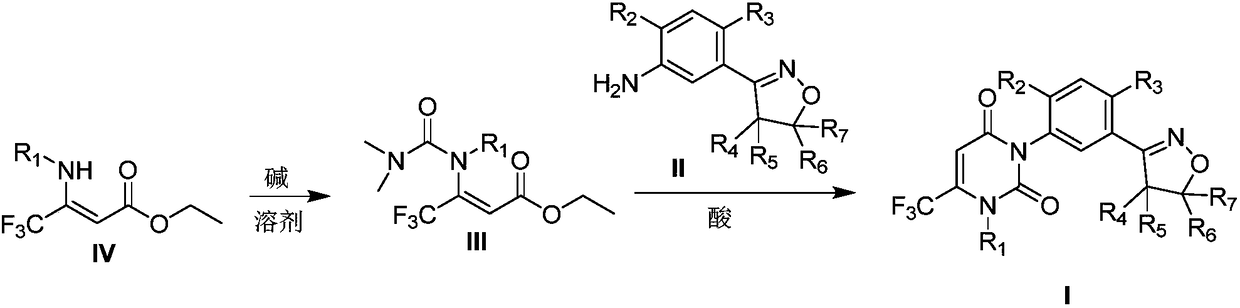
Preparation method for isoxazoline-containing uracil compound
A technology for oxazoline uracil and compound is applied in the field of preparation of isoxazoline uracil-containing compounds, and can solve problems such as hindering the popularization and application of compounds, difficult industrial production of compounds, and inability of preparation methods to meet industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
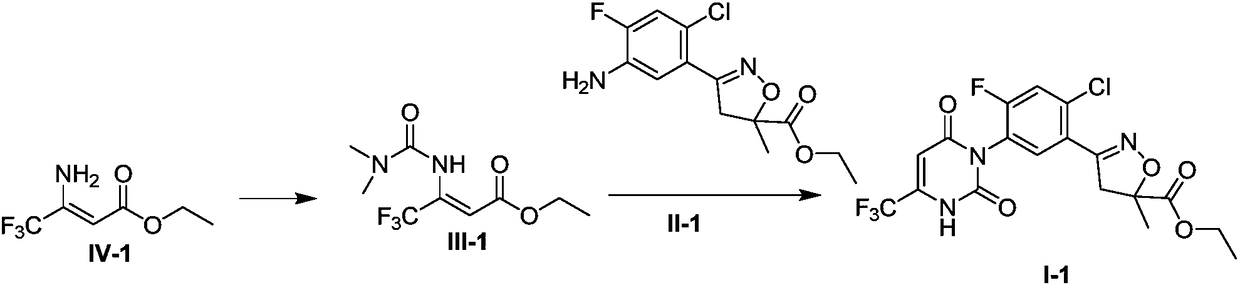
[0065] The synthesis (1) of embodiment 1 compound I-1
[0066]
[0067] Sodium hydride (60%, 80g, 2mol) was added to 500mL DMF, and a mixed solution of trifluoroamino crotonate (compound IV-1, 98%, 186g, 1mol) and 120mL DMF was added dropwise under ice cooling for 1.5 hours After completion of the dropwise addition, warm up to room temperature (20-25°C) and stir for 1 hour, add dimethylcarbamoyl chloride (97%, 166g, 1.5mol) dropwise under ice-bath conditions, stir at room temperature for 3-4 hours, TLC monitors that the reaction is complete . Evaporate most of the DMF under reduced pressure, pour it into 500mL saturated aqueous sodium bicarbonate solution, and extract twice with 600mL ethyl acetate successively, combine the ethyl acetate phase, add 100g diatomaceous earth to the Buchner funnel to filter, and decompress After distillation, 238 g of oil was obtained, namely compound III-1, with a purity of 96.7% (HPLC normalized content), and a yield of 90.6%.
[0068] 238g...
Embodiment 2
[0069] The synthesis (2) of embodiment 2 compound I-1
[0070] Potassium tert-butoxide (98%, 172g, 1.5mol) was added to 650mL DMF, followed by trifluoroamino crotonate (compound IV-1, 98%, 186g, 1mol), stirred at room temperature (20-25°C) for 1 After 1 hour, dimethylcarbamoyl chloride (97%, 166 g, 1.5 mol) was added dropwise, stirred at room temperature for 5 hours, and the reaction was complete as monitored by TLC. Evaporate most of the DMF under reduced pressure, pour it into 500mL water, and extract twice with 600mL ethyl acetate, combine the ethyl acetate phase, add 100g of diatomaceous earth to the Buchner funnel, filter, and distill under reduced pressure to obtain 228g of oil That is compound III-1, the purity is 95% (HPLC normalized content), and the yield is 85.3%.
[0071]228g of compound III-1 (96.7%, 0.853mol) and 270g of compound II-1 (synthesized with reference to WO2016095768, 90%, 0.81mol) were successively added to a reaction flask containing 600ml of acetic...
Embodiment 3
[0072] The synthesis (1) of embodiment 3 compound 1-2
[0073]
[0074] Sodium hydride (60%, 80g, 2mol) was added in 500mL DMF, and a mixed solution of trifluoromethylamino crotonate (compound IV-2, 97%, 203g, 1mol) and 120mL DMF was added dropwise under ice-cooling, and after 1.5 Add dropwise after 1 hour, raise to room temperature (20-25°C) and stir for 1 hour, add dimethylcarbamoyl chloride (97%, 166g, 1.5mol) dropwise under ice-bath conditions, stir at room temperature for 3-4 hours, monitor the reaction by TLC completely. Evaporate most of the DMF under reduced pressure, pour it into 500mL saturated aqueous sodium bicarbonate solution, and extract twice with 600mL ethyl acetate successively, combine the ethyl acetate phase, add 100g diatomaceous earth to the Buchner funnel to filter, and decompress The distillation obtained 253g of oily substance which was compound III-2, with a purity of 97.2% (HPLC normalized content), and a yield of 91.8%.
[0075] 253g of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com