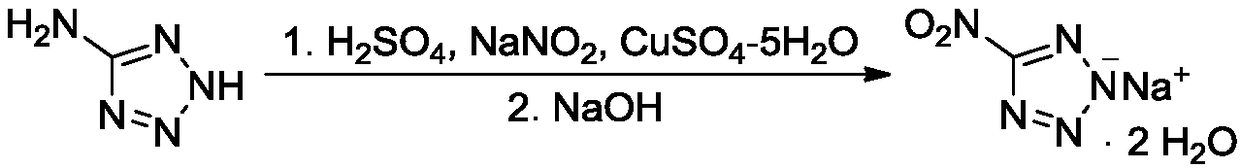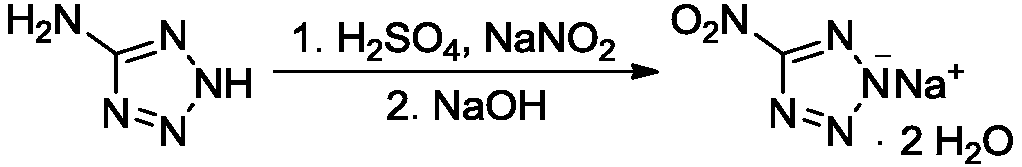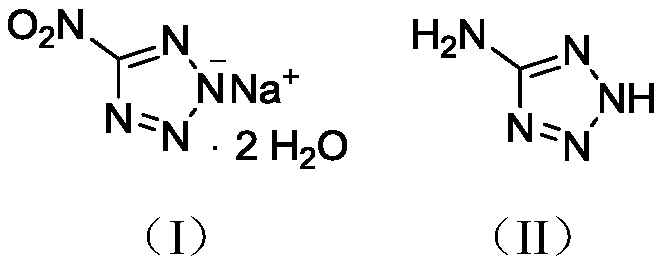Synthesis method of 5-nitrotetrazole sodium salt dihydrate
A technology of sodium nitrotetrazolium and dihydrate, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of cumbersome, complicated process operation, and low product yield, and achieve complex operation process, simple operation process, and high product yield. The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Under stirring, add 5.15g (60.6mmol) of 5-aminotetrazole, 80mL of distilled water, and 3g of concentrated sulfuric acid with a mass percentage of 95% to 98% into the reaction flask in sequence at 20°C to 25°C, heat up to 40°C, and stir Dissolve and set aside; under stirring, add 12.55g (181.8mmol) sodium nitrite into 80mL distilled water, stir to dissolve, add the above-mentioned sulfuric acid aqueous solution of 5-aminotetrazole dropwise, control the temperature of the system not higher than 30°C, add dropwise After completion, stir for 10 minutes, raise the temperature to 65°C to 70°C for 2 hours, add 3g of sodium hydroxide, stir to dissolve, distill off the water under reduced pressure, add 360mL acetone to the distillation bottle and reflux for 2 hours, remove insoluble matter by filtration, and evaporate the filtrate under reduced pressure Acetone and air-dried to obtain 10.13 g of 5-nitrotetrazole sodium salt dihydrate, with a yield of 96.7%.
[0018] Structure Id...
Embodiment 2
[0027] Under stirring, at 20°C to 25°C, 5.15g (60.6mmol) of 5-aminotetrazole, 80mL of distilled water, and 1.8g of concentrated sulfuric acid with a mass percentage of 95% to 98% were successively added to the reaction flask, and the temperature was raised to 40°C. Stir to dissolve and set aside; under stirring, add 10.46g (151.5mmol) sodium nitrite to 80mL distilled water, stir to dissolve, add the above-mentioned sulfuric acid aqueous solution of 5-aminotetrazole dropwise, control the temperature of the system not higher than 30°C, drop After the addition, stir for 10 minutes, raise the temperature to 65°C to 70°C for 2 hours, add 1.8g of sodium hydroxide, stir to dissolve, distill off the water under reduced pressure, add 206mL of acetone to the distillation bottle and reflux for 2 hours, filter to remove insoluble matter, and depressurize the filtrate The acetone was distilled off and air-dried to obtain 5.09 g of 5-nitrotetrazolium sodium salt dihydrate with a yield of 48....
Embodiment 3
[0029] Under stirring, at 20°C to 25°C, 5.15g (60.6mmol) of 5-aminotetrazole, 80mL of distilled water, and 4.12g of concentrated sulfuric acid with a mass percentage of 95% to 98% were successively added to the reaction flask, and the temperature was raised to 40°C. Stir to dissolve and set aside; under stirring, add 14.64g (212.1mmol) sodium nitrite into 80mL distilled water, stir to dissolve, add the above-mentioned sulfuric acid aqueous solution of 5-aminotetrazole dropwise, control the temperature of the system not higher than 30°C, drop After the addition, stir for 10 minutes, raise the temperature to 65°C-70°C for 2 hours, add 4.12g of sodium hydroxide, stir to dissolve, distill off water under reduced pressure, add 412mL of acetone to the distillation bottle and reflux for 2 hours, filter to remove insoluble matter, and depressurize the filtrate The acetone was distilled off and air-dried to obtain 8.68 g of 5-nitrotetrazolium sodium salt dihydrate with a yield of 82.8%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com