New crystal from of pentostatin, and preparation method and application of same
A pentostatin and crystal form technology, which is applied in the field of chemical pharmacy, can solve the problems of unfavorable crystal form stability preparation storage stability, poor fluidity of needle-like crystals, affecting the operability of preparations, etc., to reduce the decline in curative effect Effects of risk and safety risks, reduced sieving time, improved uniformity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 0.2g crude pentostatin (HPLC purity> 95%) dissolved in 20mL methanol, heated to 50°C, dissolved, filtered, added 80ml methyl acetate with stirring, crystallized at 20°C for 6h, controlled the stirring speed at 170rpm / min, filtered, and dried under vacuum at 35°C to obtain 0.08g Lump crystals are easy to filter, and the purity detected by HPLC is 99.7%.
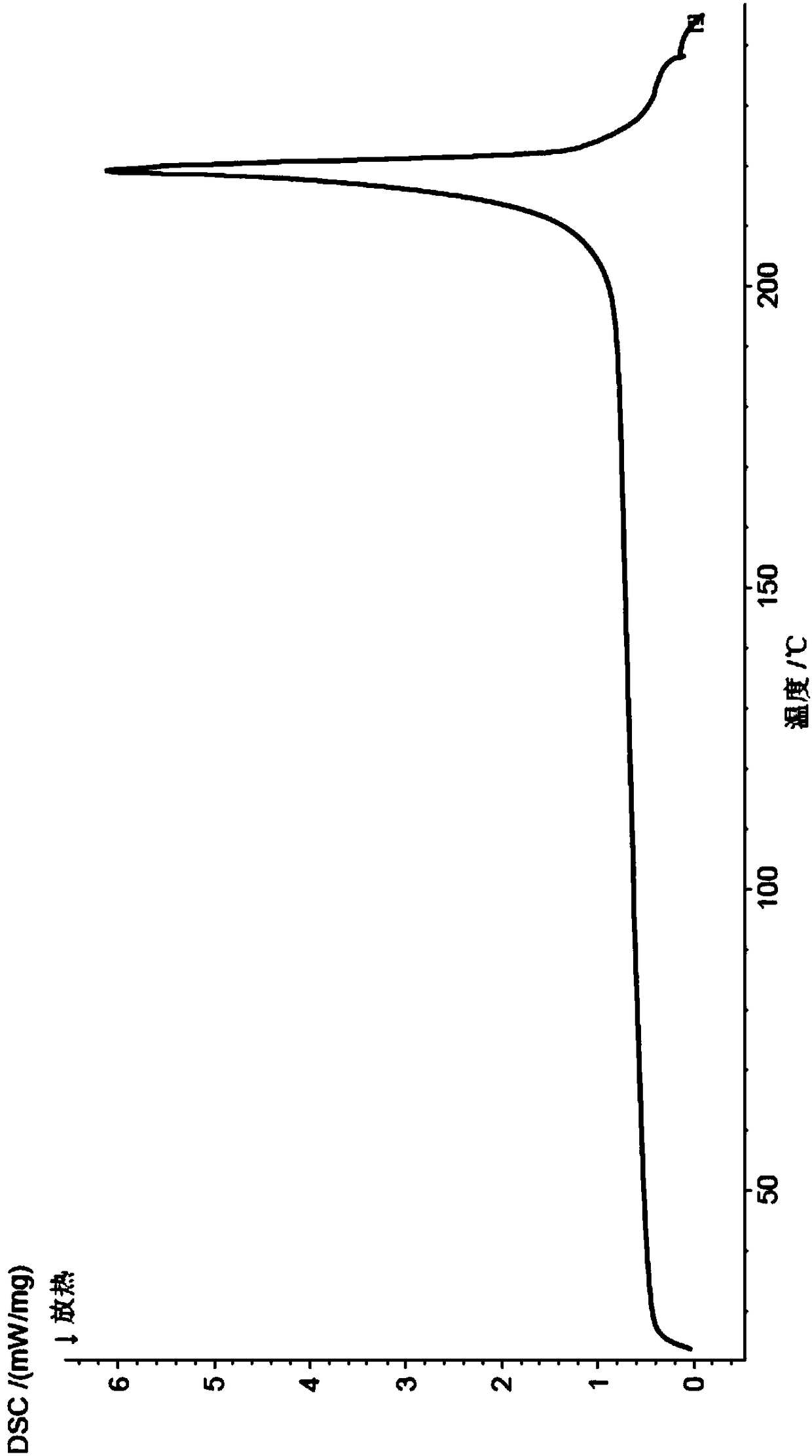
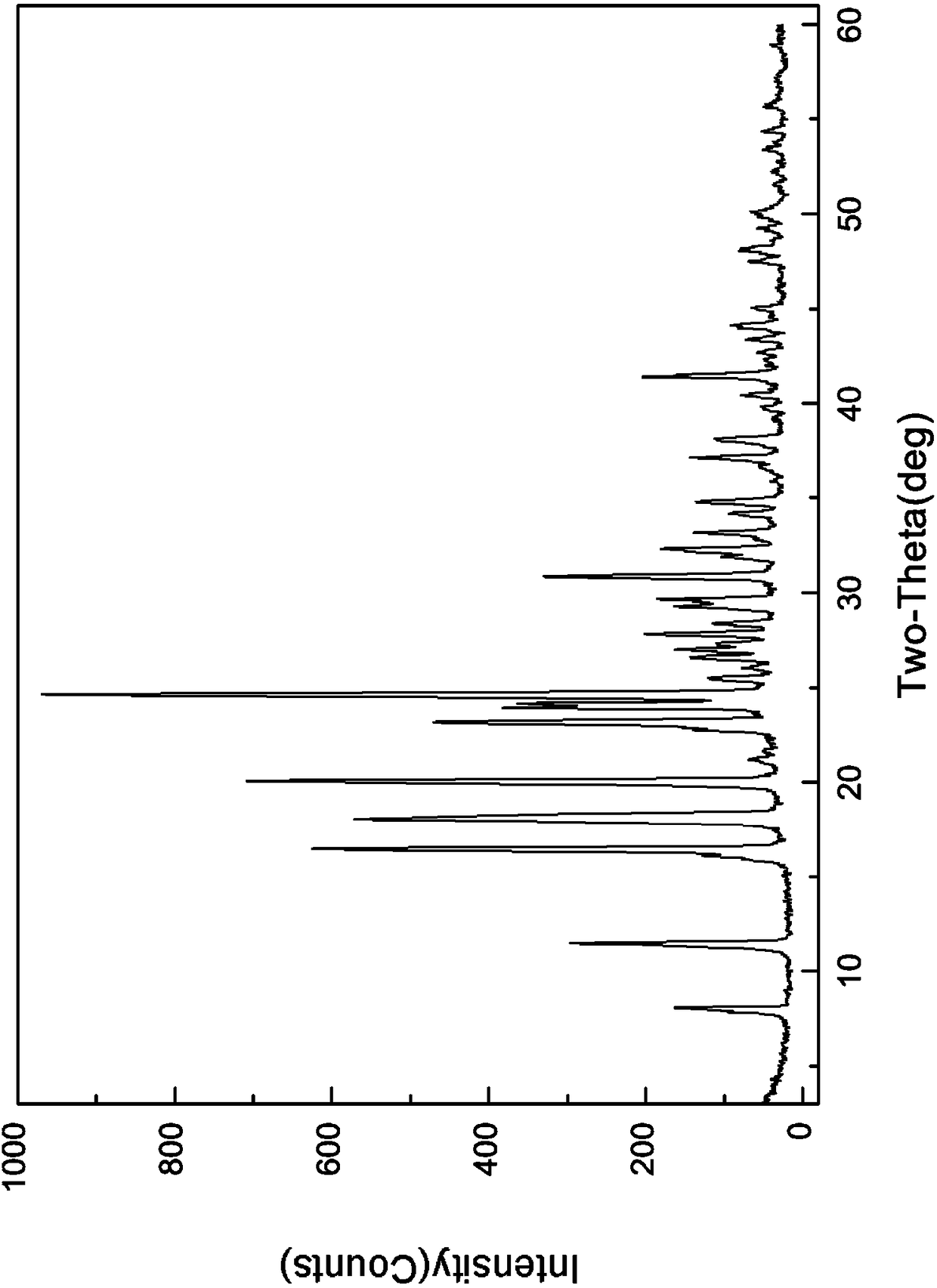
[0049] The X-ray powder diffraction and DSC spectra of this crystal are detailed in Figure 1-2 In the present invention, it is named pentostatin crystal form I.
Embodiment 2
[0051] 0.2g crude pentostatin (HPLC purity> 95%) dissolved in 10mL methanol, warmed to 60℃, dissolved, filtered, added 60ml methyl acetate with stirring, crystallized at 25℃ for 24h, controlled the stirring speed at 170rpm / min, filtered and dried under vacuum at 35℃ to obtain 0.11g Lump crystals are easy to filter, and the purity detected by HPLC is 99.5%. After measuring the X-ray powder diffraction pattern (XRD), it is confirmed that it is pentostatin crystal form I.
Embodiment 3
[0053] 0.2g crude pentostatin (HPLC purity> 95%) dissolved in 12mL methanol, heated to 55℃, dissolved, filtered, added 120ml methyl acetate with stirring, crystallized at 30℃ for 8h, controlled the stirring speed at 170rpm / min, filtered, and dried under vacuum at 35℃ to obtain 0.14g Lump crystals are easy to filter, and the purity detected by HPLC is 99.1%. After measuring the X-ray powder diffraction pattern (XRD), it is confirmed that it is pentostatin crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com