Amplification method of umbilical cord mesenchymal stem cells and application of umbilical cord mesenchymal stem cells in arthritis
A technology of mesenchymal stem cells and umbilical cords, applied in the field of osteoarthritis treatment, can solve the problems of limited expansion capacity and achieve the effect of fast expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Isolation and culture of mesenchymal stem cells. In the operating room, the umbilical cord is removed, stored in sterile saline, and transported to the laboratory on a cold chain. On the 100-level ultra-clean workbench of the GMP laboratory, use sterile surgical instruments to peel off Wharton's jelly, add it to 10 times the volume of trypsin digestion solution (0.5% trypsin + 0.2% EDTA), and keep at 37 degrees Lower digestion 2h. Then add 1mg / mL type I collagen and continue to digest for 1h. Pass the above suspension through a 200-mesh sieve, blow and filter. The above cell suspension was mixed with the culture medium, resuspended, added to a T25 cell culture flask, and cultured in a 37°C, 5% carbon dioxide incubator until generation P1.
Embodiment 2
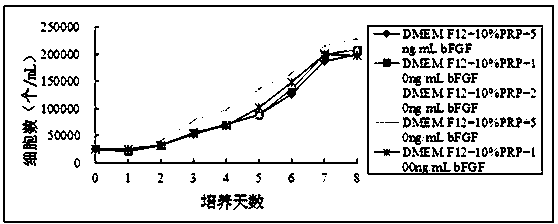
[0041] Example 2: Divide the experiment into five groups, Group A (DMEM / F12+ 10%PRP+5ng / mL bFGF), Group B (DMEM / F12+ 10%PRP+10ng / mL BFGF), Group C (DMEM / F12+ 10 %PRP + 20ng / mL bFGF), group D (DMEM / F12+10%PRP + 50ng / mL bFGF), group E (DMEM / F12+ 10%PRP + 100ng / mL bFGF).
[0042] After centrifugation, the P1 generation cells were resuspended in DMEM / F12 medium containing 10% PRP, and the density was adjusted to 5×10 4 cells / mL, inoculate five 24-well plates at 500 μL / well. According to the grouping, in the corresponding wells, add different concentrations of bFGF, 24 wells in each group. Subsequently, five 24-well plates were placed in a 37-degree, 5% carbon dioxide incubator for cultivation, and were cultured at 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, a total of 8 time points , cells from 3 wells in each group were taken at each time point for cell counting.
[0043] figure 1 The experimental results showed that the cells in group D proliferated the fastest. It shows that t...
Embodiment 3
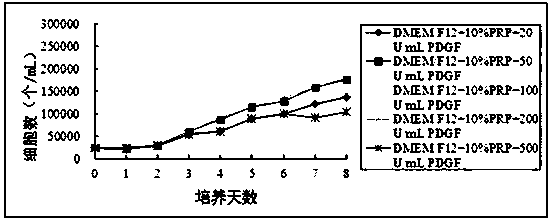
[0044] Example 3: Divide the experiment into five groups, group A (DMEM / F12+ 10%PRP + 20U / mL PDGF), group B (DMEM / F12+ 10%PRP + 50U / mL PDGF), group C (DMEM / F12+ 10 %PRP + 100U / mL PDGF), group D (DMEM / F12+10%PRP + 200U / mL PDGF), group E (DMEM / F12+ 10%PRP + 500U / mL PDGF).
[0045] After centrifugation, the P1 generation cells were resuspended in DMEM / F12 medium containing 10% PRP, and the density was adjusted to 5×10 4 cells / mL, inoculate five 24-well plates at 500 μL / well. According to the grouping, different concentrations of PDGF were added to the corresponding wells, 24 wells in each group. Subsequently, five 24-well plates were placed in a 37-degree, 5% carbon dioxide incubator for cultivation, and were cultured at 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, a total of 8 time points , cells from 3 wells in each group were taken at each time point for cell counting.
[0046] figure 2 The experimental results showed that the cell proliferation in group C was the fastest. It...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com