Quasi-solid state lithium ion conductive electrolyte and preparation method and application thereof
A lithium-ion, quasi-solid-state technology, applied in the field of new lithium battery solid-state electrolytes, can solve the problems of interface stability and affect the cycle stability of lithium anodes, and achieve excellent mechanical properties and chemical/electrochemical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
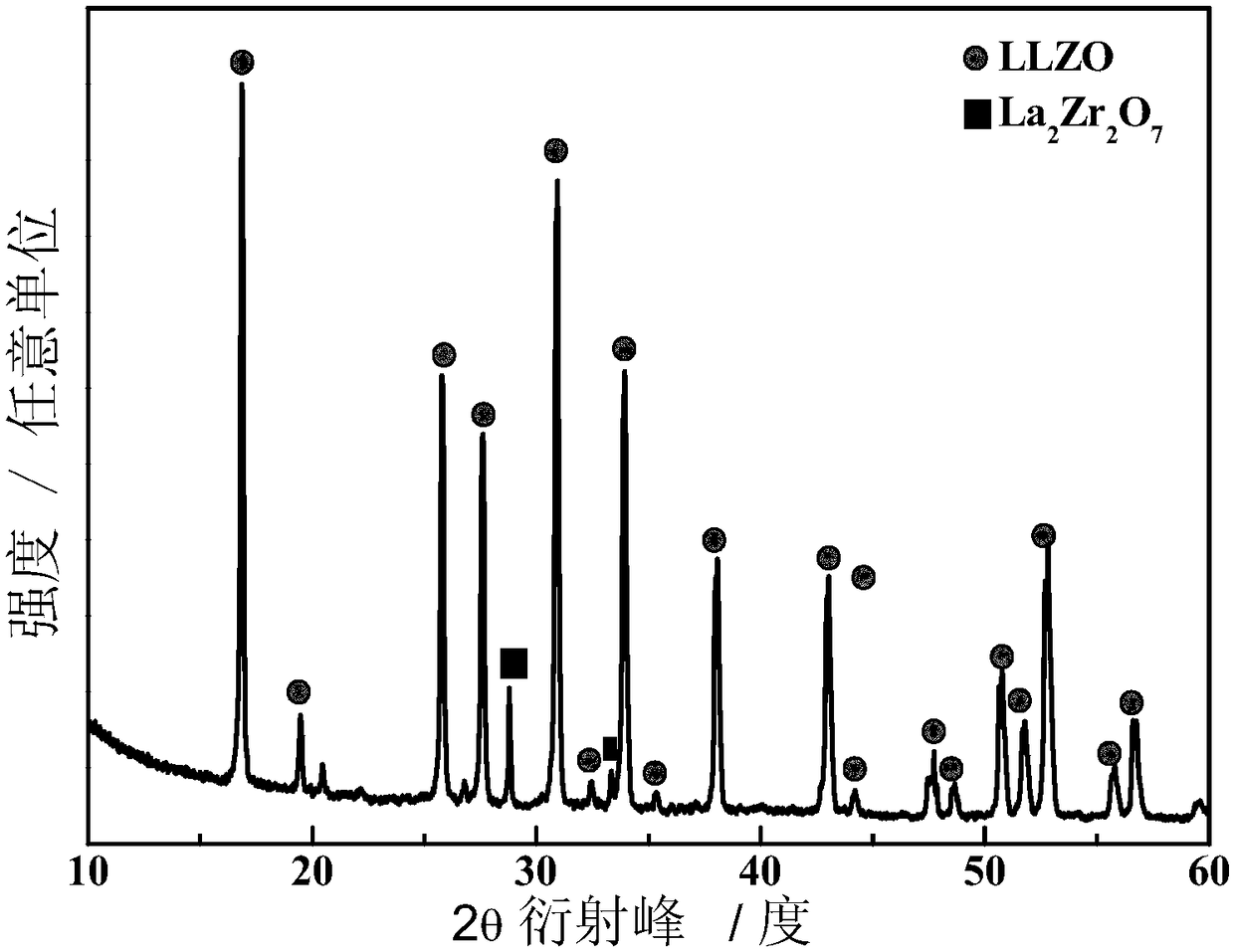
[0064] The ceramic electrolyte was prepared by the sol-gel method, 3.27 grams of zirconium n-propoxide was dissolved in 0.71 grams of ethyl acetoacetate, and the solution A was obtained by thorough stirring; 2.41 grams of LiNO 3 and 6.49 g La(NO 3 ) 3 ·6H 2 O was dissolved in 7.53 g of absolute ethanol to obtain solution B; solution B was added to solution A, and stirred at 40 ° C for 2 hours to obtain a gel; the above gel was freeze-dried to obtain a precursor, and then the precursor Roasting under the atmosphere, the heating rate is 2°C / min, the firing temperature is 600°C, and the firing time is 8 hours, then the powder obtained by firing is pressed at 10MPa, and the ceramic electrolyte is obtained by repeated firing with the above firing process; Vinyl fluoride-hexafluoropropylene and 0.8 g of polypropylene carbonate were placed in 50 mL of methylpyrrolidone, stirred thoroughly at 80 ° C to obtain a homogeneous solution, and then 0.32 g of ceramic electrolyte and 0.32 g ...
Embodiment 2
[0080] The ceramic electrolyte was prepared by the sol-gel method, 3.22 grams of zirconium n-propoxide was dissolved in 0.71 grams of ethyl acetoacetate, and the solution A was obtained by thorough stirring; 2.41 grams of LiNO 3 and 6.33 g La(NO 3 ) 3 ·6H 2 O was dissolved in 7.53 g of absolute ethanol to obtain solution B; solution B was added to solution A, and stirred at 40 ° C for 2 hours to obtain a gel; the above gel was freeze-dried to obtain a precursor, and then the precursor Roasting under the atmosphere, the heating rate is 2°C / min, the firing temperature is 650°C, and the firing time is 6 hours, then the powder obtained by firing is pressed at 10MPa, and the ceramic electrolyte is obtained by repeated firing with the above firing process; 1.12 grams of poly Vinyl fluoride-hexafluoropropylene and 0.48 g of polypropylene carbonate were placed in 50 mL of methylpyrrolidone, stirred thoroughly at 80 ° C to obtain a homogeneous solution, and then 0.32 g of ceramic ele...
Embodiment 3
[0084] The ceramic electrolyte was prepared by the sol-gel method, and 3.32 g of zirconium n-propoxide was dissolved in 0.71 g of ethyl acetoacetate, and the solution A was obtained by thorough stirring; 2.41 g of LiNO 3 and 6.65 g La(NO 3 ) 3 ·6H 2 O was dissolved in 7.53 g of absolute ethanol to obtain solution B; solution B was added to solution A, and stirred at 40 ° C for 2 hours to obtain a gel; the above gel was freeze-dried to obtain a precursor, and then the precursor Roasting under the atmosphere, the heating rate is 2°C / min, the firing temperature is 550°C, and the firing time is 12 hours, then the powder obtained by firing is pressed at 10MPa, and the ceramic electrolyte is obtained by repeated firing with the above firing process; Vinyl fluoride-hexafluoropropylene and 1.12 grams of polypropylene carbonate were placed in 50 mL of methylpyrrolidone, stirred thoroughly at 80 ° C to obtain a homogeneous solution, and then 0.32 grams of ceramic electrolyte and 0.32 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com