Dextral lipoic acid-lysine salt enteric tablet and preparation method thereof
A technology of lipoic acid lysine salt and lysine salt, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of reducing the content, low melting point and stable It can prevent easy polymerization, stabilize blood drug concentration, and improve thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
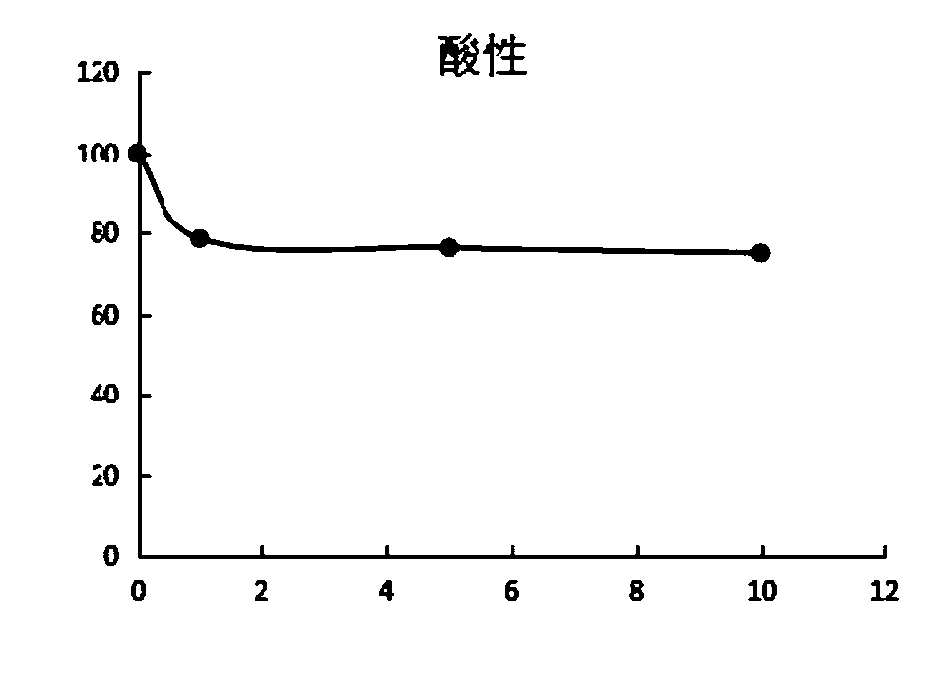
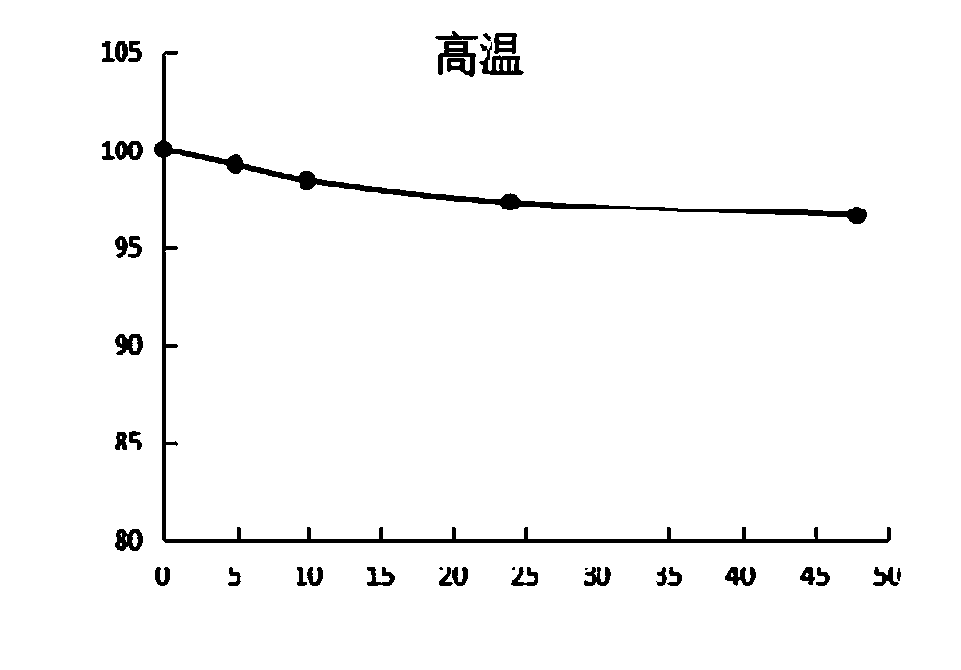
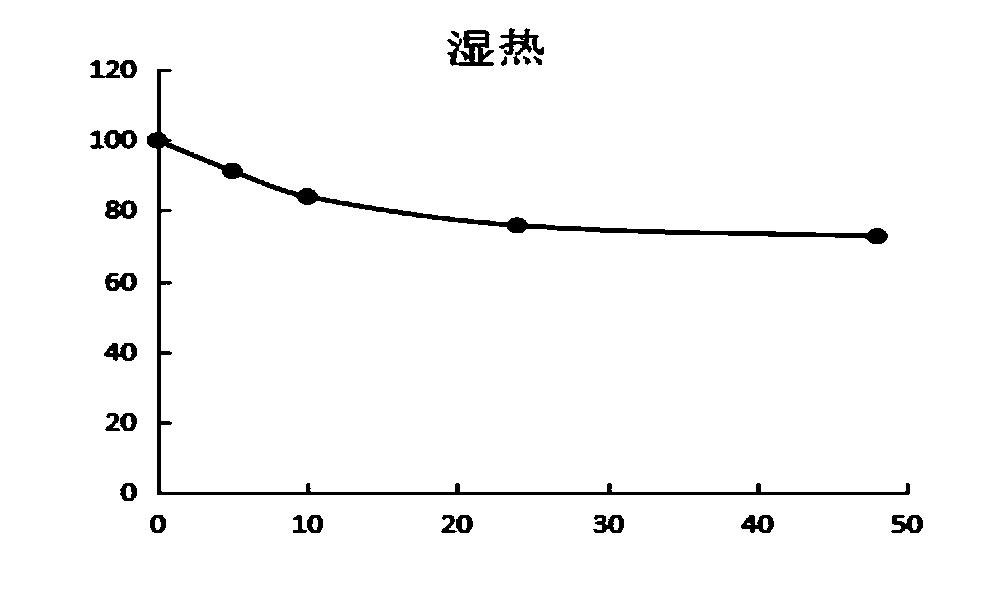
[0058] The present invention tests the stability data of the above-mentioned D-lipoic acid lysine salt under acidic, high temperature, humid heat and light conditions. see details Figure 1~4 , It is unstable under acidic conditions, and the content will drop obviously after being placed for 1 hour, and the content will drop less after continued storage, mainly due to the rapid polymerization after contact with acid, which will cause the content to drop, and the content will no longer drop significantly after polymerization. Under hot and humid conditions, the content gradually decreased over time and was obvious. When placed under high temperature and dry conditions, the content decreased relatively little, indicating that D-lipoic acid lysine was unstable under conditions of heat, humidity, high temperature and light, especially in acidic and humid conditions. Polymerization is more likely to occur under these conditions.
Embodiment 2
[0060] 1. Screening of enteric-coated carriers
[0061] In this experiment, M-type HPMCAS, L-type HPMCAS, HPMCP, Eudragit L100 and Eudragit L100-55 with a dissolved pH between 5 and 6 were selected as enteric-coated materials, and the effects of different enteric-coated materials on the extrusion process and drug release in vitro were investigated. Impact. Among them, M-type HPMCAS has the smallest torque during extrusion, and the color of the extruded product is the most similar to that of the raw material drug, and there is no polymerization phenomenon in the medium of pH 1.0, indicating that the enteric-coated material has a better wrapping effect on the main drug , preventing the ring-opening polymerization of the main drug. Therefore, M-type HPMCAS is preferred as the enteric carrier material.
[0062] 2. Screening of enteric-coated carrier ratio
[0063] The ratio of carrier to drug usually affects the feasibility of extrusion, the dissolution and stability of the dru...
Embodiment 3
[0066] The screening of embodiment 3 fillers
[0067] The filler mainly plays the role of increasing the volume and facilitating its molding in the tablet, and the filler is selected from one or more of lactose, microcrystalline cellulose, mannitol, and calcium hydrogen phosphate. Considering the particularity of medication for diabetic patients, sugars such as lactose should not be used as fillers. Similarly, mannitol will increase the burden on the kidneys of diabetic patients, so it is also not suitable for use. Microcrystalline cellulose, which is not absorbed in the body, has good compressibility and fluidity, and is widely used in powder direct compression and wet granulation compression, and has a certain capillary action, allowing water to enter quickly after contact with water The tablet core breaks the bonds between the particles and contributes to the rapid disintegration of the tablet. Therefore, microcrystalline cellulose is preferably used as the filler of the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com