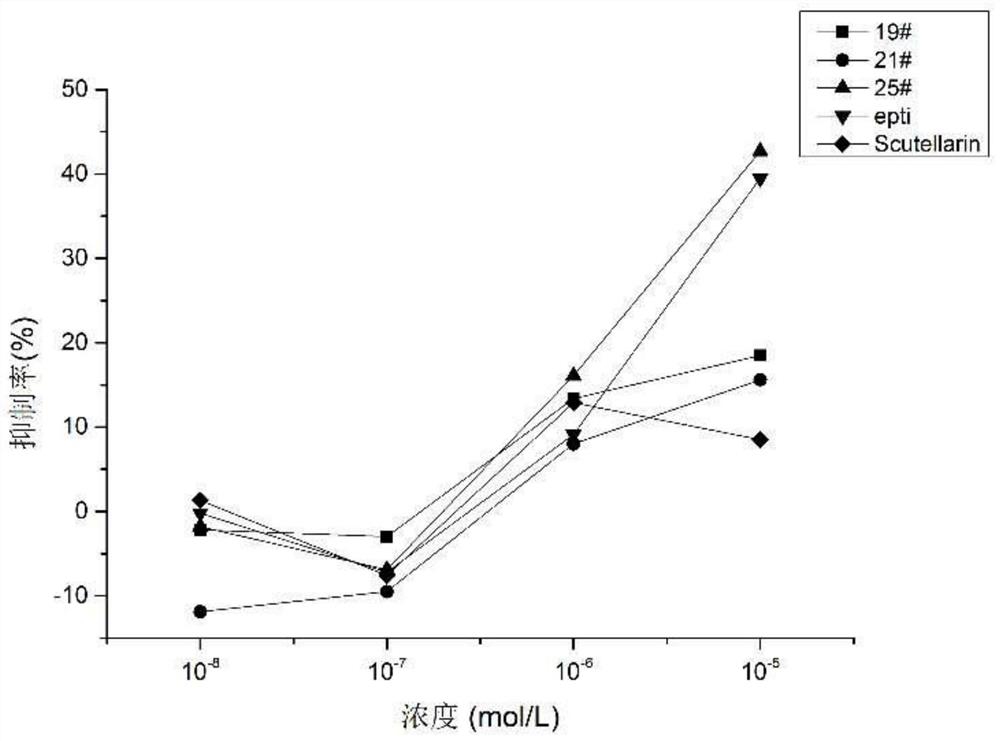
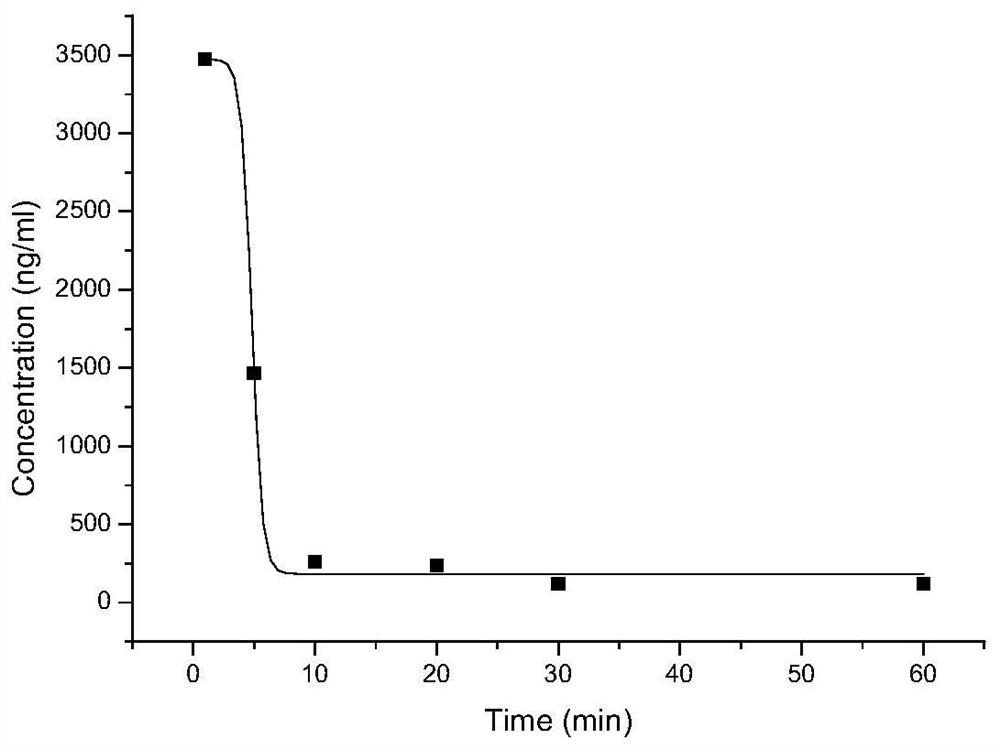
Preparation and purification method and application of a kind of peptidomimetic compound
A compound and peptide technology, applied in the field of medicine, can solve problems such as inconvenient clinical administration of injections, joint swelling, anaphylactic shock, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
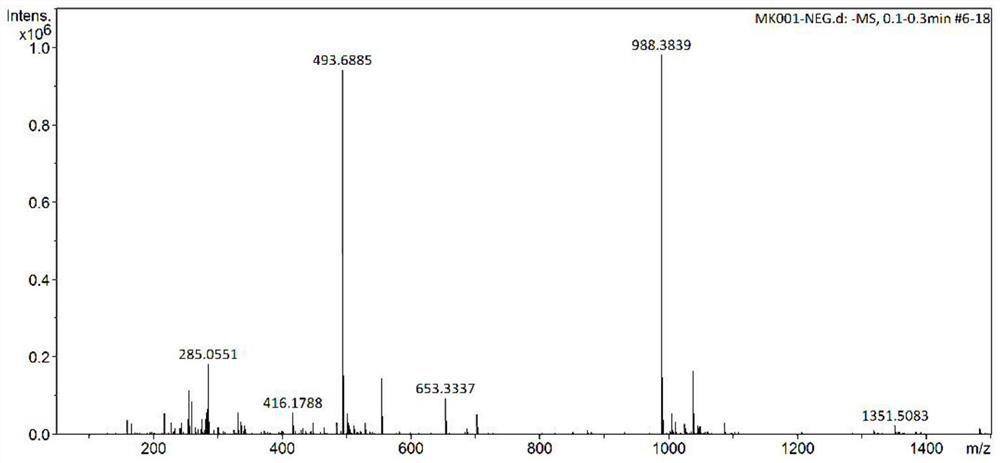
[0033] Example 1 Anti-platelet peptidomimetic compound Scutellarin-HomoArg-Gly-Asp-Trp-NH 2 Synthesis
[0034] 1.1 Preparation of Fmoc-Linker-Amide Resin
[0035] Weigh 50 g (60 mmol) of Amide Resin resin into a 2L solid-phase synthesis reaction column. Wash the resin once with 400ml DMF, swell the resin with 500ml DCM for 20min, and remove the solvent. 400ml DMF washed the resin 3 times.
[0036] Weigh 80.9g (150mmol) of Fmoc-linker and 24.3g (180mmol) of HOBt into a dry 500ml wide-mouth Erlenmeyer flask. Add about 150ml of DMF solvent to dissolve and place in ice-water bath to cool for 2min, add DIC 30ml (195mmol) to activate for 3min, avoid water vapor. The activated amino acid was added to the swollen resin to react for 2 hours, and the reaction solution was sucked away. Wash the resin 3 times with 400ml DMF, 2min each time.
[0037] Join Ac 2 O 28ml (300mmol), DIPEA 10.7ml (60mmol), DMF 400ml mixed solution sealed for 3h, the resin was washed four times with DMF 50...
Embodiment 2
[0070] Example 2 Anti-platelet peptidomimetic compound Scutellarin-HomoArg-Gly-Asp-NH 2 Synthesis
[0071] 2.1 Preparation of Fmoc-Linker-Amide Resin
[0072] Weigh 50 g (60 mmol) of Amide Resin resin into a 2L solid-phase synthesis reaction column. Wash the resin once with 400ml DMF, swell the resin with 500ml DCM for 20min, and remove the solvent. The resin was washed three times with 400ml DMF.
[0073] Weigh 80.9g (150mmol) of Fmoc-linker and 24.3g (180mmol) of HOBt into a dry 500ml wide-mouth Erlenmeyer flask. Add about 150ml of DMF solvent to dissolve and place it in an ice-water bath to cool for 2 minutes, add 30ml of DIC (195mmol) to activate for 3 minutes, and avoid water vapor. The activated amino acid was added to the swollen resin to react for 2 hours, and the reaction solution was sucked away. Wash the resin 3 times with 400ml DMF, 2min each time.
[0074] Join Ac 2 O 28ml (300mmol), DIPEA 10.7ml (60mmol), DMF 400ml mixed solution sealed for 3h, the resin w...
Embodiment 3
[0102] Example 3 Anti-platelet peptidomimetic compound Scutellarin-HomoArg-Gly-Asp-Trp-Pro-NH 2 Synthesis
[0103] 3.1 Preparation of Fmoc-Linker-Amide Resin
[0104] Weigh 50 g (60 mmol) of Amide Resin resin into a 2L solid-phase synthesis reaction column. Wash the resin once with 400ml DMF, swell the resin with 500ml DCM for 20min, and remove the solvent. The resin was washed three times with 400ml DMF.
[0105] Weigh 80.9g (150mmol) of Fmoc-linker and 24.3g (180mmol) of HOBt into a dry 500ml wide-mouth Erlenmeyer flask. Add about 150ml of DMF solvent to dissolve and place it in an ice-water bath to cool for 2 minutes, add 30ml of DIC (195mmol) to activate for 3 minutes, and avoid water vapor. The activated amino acid was added to the swollen resin to react for 2 hours, and the reaction solution was sucked away. Wash the resin 3 times with 400ml DMF, 2min each time.
[0106] Join Ac 2 O 28ml (300mmol), DIPEA 10.7ml (60mmol), DMF 400ml mixed solution sealed for 3h, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com