A kind of benzoxadiazole pH fluorescent probe, preparation method and application
A technology of benzoxadiazoles and fluorescent probes, applied in the field of applied biology, can solve the problems of long synthesis time and inconvenient synthesis, and achieve the effects of short time consumption, low toxicity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of fluorescent probes includes the following experimental steps:
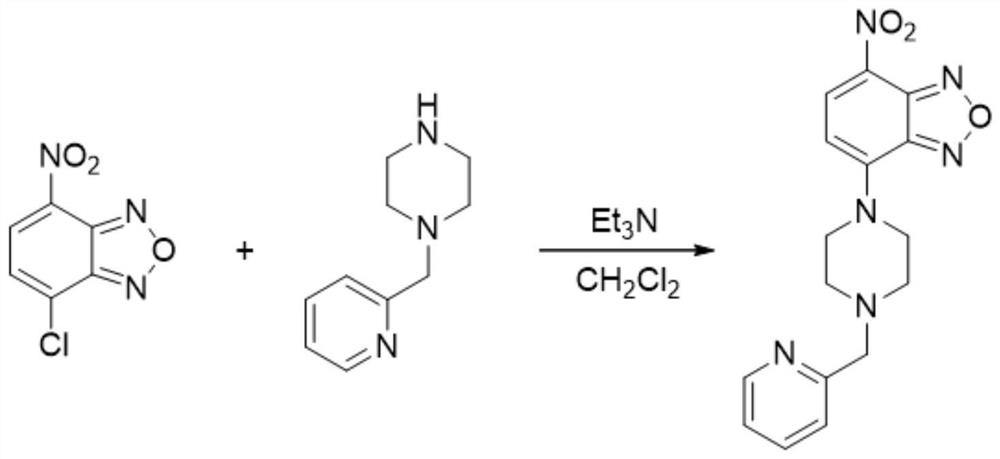
[0035] (1) Dissolve 4-chloro-7-nitrobenzofurazan (200mg, 1mmol), 1-[(2-pyridyl)methyl]piperazine (177mg, 1mmol) and triethylamine (0.5mL) In 5mL of anhydrous dichloromethane, the reaction mixture was stirred at room temperature for 1 hour, and the synthetic reaction equation of the fluorescent probe was as follows: figure 1 shown;
[0036] (2) The reaction solution was rotary evaporated, and the filtrate was purified by column, wherein the volume ratio of petroleum ether and ethyl acetate was 4:1 to obtain a yellow solid (296 mg, yield 87%).
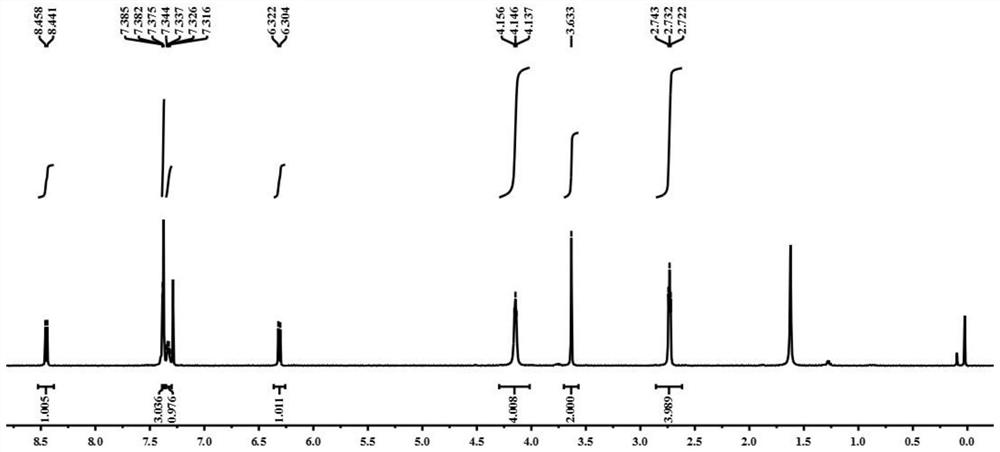
[0037] The nuclear magnetic resonance H spectrum of gained fluorescent probe is: 1 H NMR (500MHz, CDCl 3 )δ (ppm): 8.45 (d, J = 8.5, 1H,), 7.39-7.37 (m, 3H), 7.34-7.32 (m, 1H), 6.31 (d, J = 9, 1H), 4.15 (t ,J=5,4H), 3.63(s,1H), 2.73(t,J=55Hz,4H); image 3 shown.
Embodiment 2
[0039] The experimental procedure of embodiment 2 is identical with the experimental procedure of embodiment 1, but in step (1) with 4-chloro-7-nitrobenzofurazan and 1-[(2-pyridyl) methyl] piperazine The mol ratio is changed to 2:1.
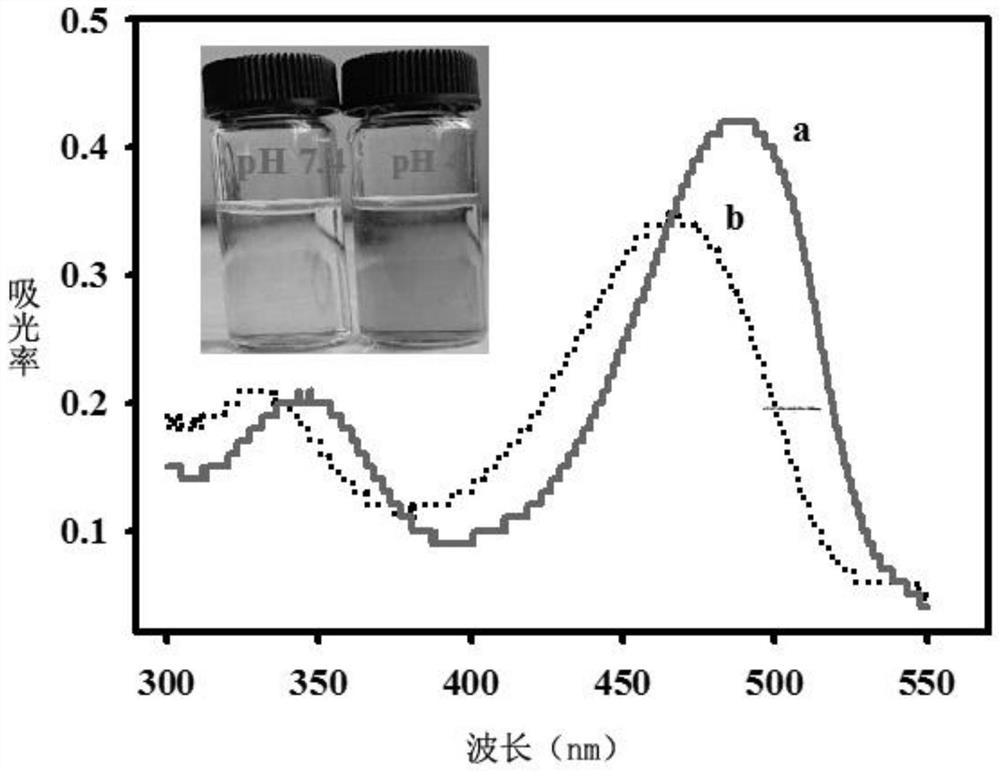
[0040] It was found through testing that the structure of the final product of Example 2 was consistent with that of Example 1, and the synthesis method of the probe of the present invention is not limited to the method described in the example.
Embodiment 3
[0042] The experimental procedure of embodiment 3 is identical with the experimental procedure of embodiment 1, but in step (1) with 4-chloro-7-nitrobenzofurazan and 1-[(2-pyridyl) methyl] piperazine The molar ratio was changed to 1:2.
[0043] It was found through testing that the structure of the final product of Example 3 was consistent with that of Example 1, and the synthesis method of the probe of the present invention is not limited to the method described in the example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com