Methyltransferase gene for glycoside compounds
A technology of methyltransferase and compound, applied in the direction of transferase, enzyme, biochemical equipment and method, etc., can solve problems such as activity reduction, and achieve the effects of enhancing stability, enhancing stability and improving transformation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
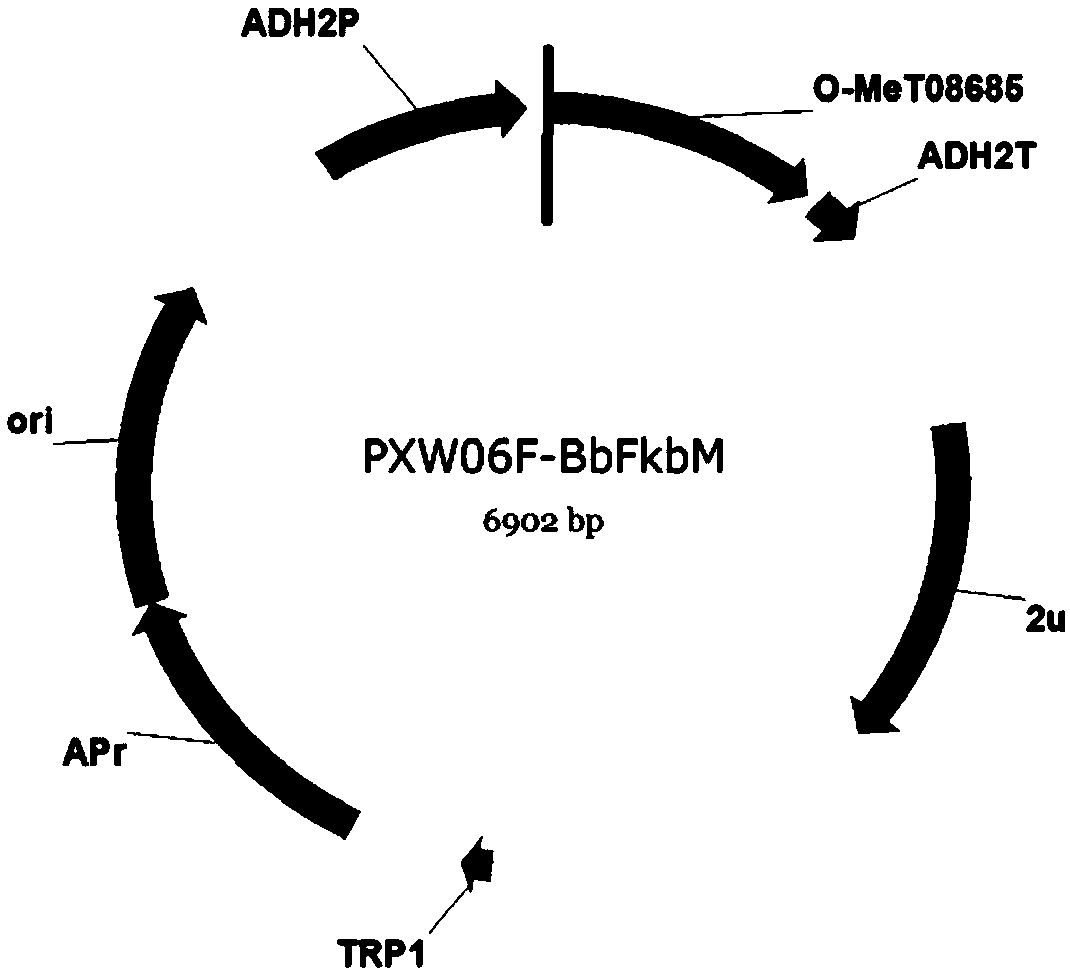
[0089] Example 1 Synthesis of O-methyltransferase gene BbFkbM, and construction of yeast expression vectors to obtain yeast transformants
[0090] Sequencing the genome of a strain of Beauveria bassiana obtained, and obtained the genome sequence of the strain. During the process of analyzing glycosyltransferases through relevant bioinformatics software, an O-methyltransferase gene was found through comparison. We named it BbFkbM. The nucleotide sequence of BbFkbM was predicted and analyzed by various software to remove intron splicing to obtain the coding region sequence of BbFkbM. Extract the RNA of Beauveria bassiana and obtain cDNA by reverse transcription, use the designed primers to amplify the coding gene of BbFkbM, recover the target fragment and send it to the sequencing company for sequencing and comparison, and obtain the glycosyltransferase shown in SEQ ID NO.1 Gene BbFkbM.
[0091] Restriction sites are introduced during synthesis. An NdeI restriction site was a...
Embodiment 2
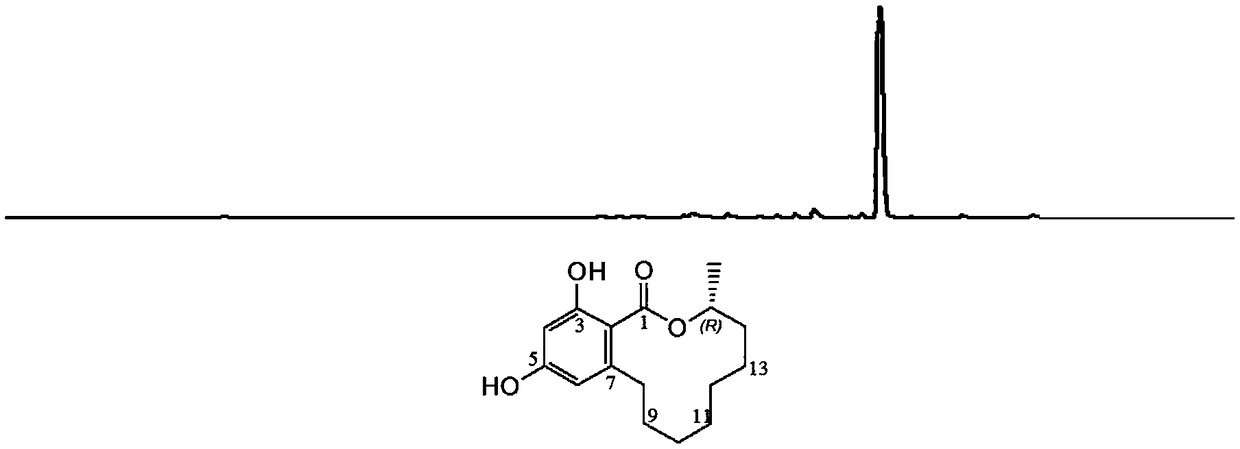
[0095] Example 2 Application of the O-methyltransferase recombinant vector PXW06F-BbFkbM to realize the modification of the glycosylated derivative of the polyketide compound Desmethyl-Lasiodiplodin (compound 1)
[0096] 1. Purpose of the experiment
[0097] The metabolites modified by glucosyltransferase and O-methyltransferase BbFkbM were separated by HPLC and their molecular structures were analyzed.
[0098] 2. Experimental method:
[0099] 1) Fermentation culture
[0100] Adopt the two-step fermentation technology, first inoculate the appropriate amount of yeast transformant cells into the corresponding 25-mL - Leu / - In Trp liquid deficient medium, culture at 30°C and 200r min-1 for about 16 hours, then add 25ml of YPD low-sugar medium, and add 5mg of pure Desmethyl-Lasiodiplodin at the same time to continue culturing for 48 hours; extract the fermentation product with ethyl acetate, ethyl acetate The ratio of ester to fermentation broth is 1:1, that is, 50ml of ethy...
Embodiment 3
[0115] Example 3 Modification of flavonoids and anthraquinones using O-methyltransferase recombinant vector PXW06F-BbFkbM
[0116] 1. Purpose of the experiment
[0117] The metabolites modified by glucosyltransferase and O-methyltransferase were separated by HPLC and their molecular structures were analyzed.
[0118] 2. Experimental method:
[0119] 1) Fermentation culture
[0120] Adopt two-step fermentation technology, first inoculate an appropriate amount of yeast transformant cells into the corresponding 25ml - Leu / - In Trp liquid deficient medium, culture at 30°C and 200r min-1 for about 16 hours, then add 25ml of YPD low-sugar medium, and at the same time add 5mg of pure product to continue culturing for 48 hours. A total of 20 flavonoid samples were used; extracted with ethyl acetate For the fermentation product, the ratio of ethyl acetate to fermentation broth is 1:1, that is, the fermentation product is extracted with 50ml of ethyl acetate; the dry extract of eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com