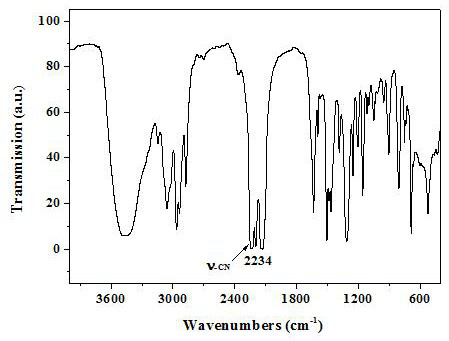
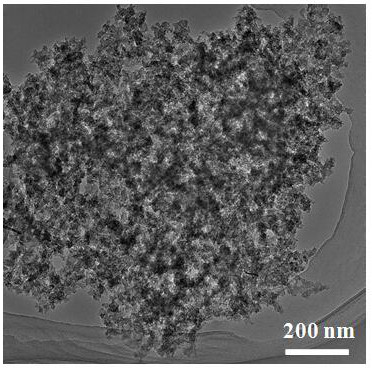
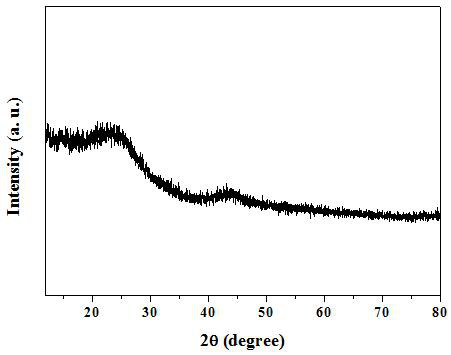
A nitrogen-doped porous carbon immobilized noble metal catalyst and its preparation method and application
A nitrogen-doped porous carbon, precious metal catalyst technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve noble metal nanoparticle aggregation, unfriendly post-treatment, low natural abundance, etc. problems, to achieve the effect of easy large-scale production and preparation, excellent catalytic performance, and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Weigh 1.0g of 1-butyl-3-methylpyridine ionic liquid and 1.0g of chitosan into a mortar and grind them evenly, mix 6.0g of KCl-ZnCl 2 The double salt (mass ratio of the two is 1:1) was added to the above system, transferred to a ball mill and continued to be ball milled to obtain a viscous solid sample.
[0037] (2) Put the obtained solid sample into a high-temperature quartz boat, raise the temperature to 600°C at a rate of 2°C / min under a nitrogen flow rate of 50 mL / min, and keep it for 1 hour before cooling to room temperature.
[0038] (3) The obtained black powder was added to 0.1mol / L hydrochloric acid solution and treated at room temperature for 1 hour, filtered, washed and dried, then added to the palladium acetate solution and ultrasonically treated at room temperature for 0.5 hours, 1.0mmol / LNaBH 4 The solution was added dropwise into the above system and stirred for 2 hours. After suction filtration, water washing and ethanol rinsing, a nitrogen-doped hi...
Embodiment 2
[0042] (1) Weigh 1.0g of ionic liquid and 2.0g of chitosan and add them into a mortar for grinding and mixing, and mix 8.0g of KCl-ZnCl 2 The double salt (mass ratio of the two) was added to the above system, transferred to a ball mill and continued to be ball milled to obtain a viscous solid sample.
[0043] (2) Put the obtained solid sample into a high-temperature quartz porcelain boat, raise the temperature to 700°C at a rate of 3°C / min under a nitrogen flow rate of 50 mL / min, and keep it for 1 hour before cooling to room temperature.
[0044] (3) The obtained black powder was added to 0.1mol / L hydrochloric acid solution and treated at room temperature for 1 hour, filtered, washed and dried, then added to the palladium acetate solution and ultrasonically treated at room temperature for 0.5 hours, 5.0mmol / LNaBH 4 The solution was added dropwise into the above system and stirred for 2 hours. After suction filtration, water washing and ethanol rinsing, a nitrogen-doped hiera...
Embodiment 3
[0047] (1) Weigh 1.0g of ionic liquid and 3.0g of chitosan into a mortar, grind and mix well, mix 9.0g of KCl-ZnCl 2 The double salt (mass ratio of the two is 1:3) was added to the above system, transferred to a ball mill and continued to be ball milled to obtain a viscous solid sample.
[0048] (2) Put the obtained solid sample into a high-temperature quartz porcelain boat, raise the temperature to 800°C at a heating rate of 5°C / min under the condition of a nitrogen flow rate of 100 mL / min, and keep it for 1 hour before cooling to room temperature.
[0049] (3) The obtained black powder was added to 0.2mol / L hydrochloric acid solution and treated at room temperature for 1 hour, filtered, washed and dried, then added to the palladium acetate solution and ultrasonically treated at room temperature for 0.5 hours, 5.0mmol / LNaBH 4 The solution was added dropwise into the above system and stirred for 2 hours. After suction filtration, water washing and ethanol rinsing, a nitrogen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com