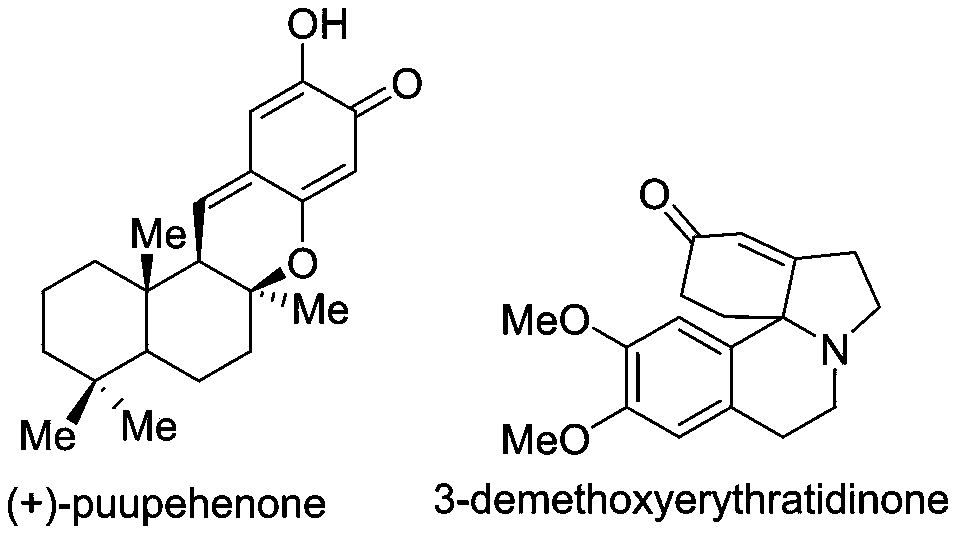
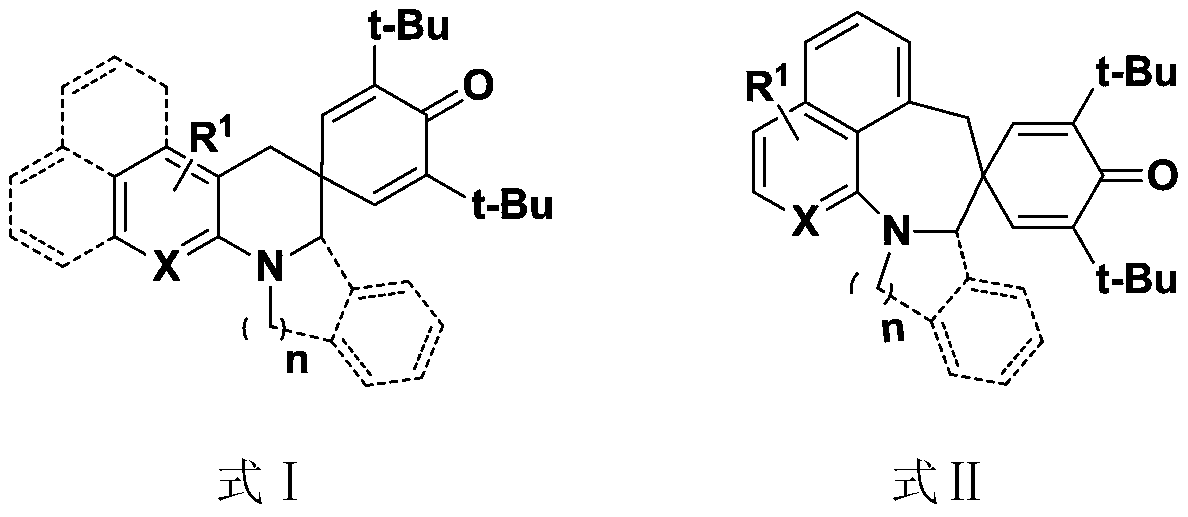
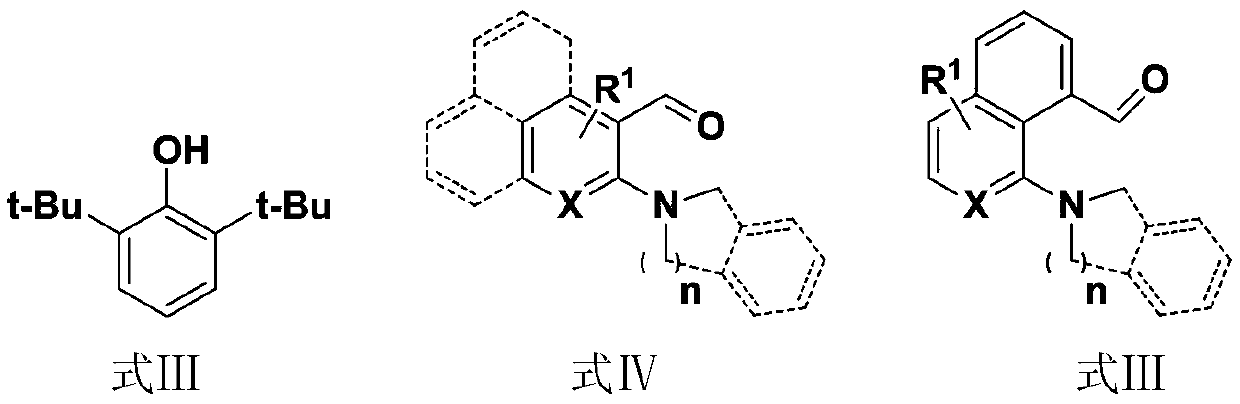
A kind of synthetic method of nitrogen heterocyclic substituted p-quinone skeleton spiro compound
A technology of spiro compound and synthesis method, which is applied in the field of synthesis of nitrogen heterocyclic substituted p-quinone skeleton spiro compound, can solve the problem of hindering dearomatization of hydrogen migration/cyclization reaction, unable to realize dearomatization of condensed ring aromatics, and not It is suitable for the efficient synthesis of quinone compounds and other problems, and achieves the effect of complete conversion of raw materials, convenient separation of products, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] take compound A 1 (2,6-di-tert-butylphenol) 0.13mmol, Compound B 1 (2-Pyrrolidinylbenzaldehyde) 0.1mmol and piperidine 0.2mmol in a reaction flask, add 1mL of toluene, stir and react at 120°C for 12h, then cool to room temperature;
[0041] Subsequently, 1 mL of hexafluoroisopropanol HFIP was added to the reaction system, and stirred at 25° C. for 10 min. After the reaction was completed, the reaction product was concentrated by rotary evaporation, and purified and separated on a silica gel column.
[0042] The isolated product was detected and analyzed, and the results of the analyzed data were as follows, the obtained product was the target product, and the yield was 60%.
[0043] Chemical formula: C 25 h 33 NO
[0044] Exact molecular weight: 363.2562
[0045] Molecular weight: 363.5450
[0046] Structural formula:
[0047] Yield: 60%
[0048] 1 H NMR (500MHz, CDCl 3 )δ7.14(t, J=7.7Hz, 1H), 6.99(d, J=7.4Hz, 1H), 6.62(t, J=7.3Hz, 1H), 6.51(d, J...
Embodiment 2
[0050] take compound A 1 (2,6-di-tert-butylphenol) 0.13mmol, compound B 1 (2-Pyrrolidinylbenzaldehyde) 0.1mmol and piperidine 0.2mmol were placed in a reaction flask, 1mL of toluene was added, and the reaction was stirred at 120°C for 12h, then cooled to room temperature, and the reaction intermediate was separated and purified;
[0051] The reaction intermediate was added into 2 mL of hexafluoroisopropanol HFIP, and stirred at 25° C. for 10 min. After the reaction was completed, the reaction product was concentrated by rotary evaporation, and purified and separated on a silica gel column.
[0052] Detection and analysis were carried out on the isolated product, and the results of the analysis data were as follows, the obtained product was the target product, and the yield was 90%.
[0053] Chemical formula: C 25 h 33 NO
[0054] Exact molecular weight: 363.2562
[0055] Molecular weight: 363.5450
[0056] Structural formula:
[0057] Yield: 90%
[0058] 1 H NMR (50...
Embodiment 3
[0061] Raw materials: 4-bromo-2-pyrrolidinylbenzaldehyde, 2,6-di-tert-butylphenol
[0062] Product: Chemical formula: C 25 h 32 BrNO
[0063] Exact molecular weight: 441.1667
[0064] Molecular weight: 442.4410
[0065] Structural formula:
[0066] Yield: 50%
[0067] 1 H NMR (500MHz, CDCl 3 )δ6.83(d, J=7.8Hz, 1H), 6.72(dd, J=7.9, 1.9Hz, 1H), 6.61(d, J=1.9Hz, 1H), 6.33(d, J=2.9Hz, 1H), 6.24(d, J=2.9Hz, 1H), 3.61(dd, J=9.5, 6.0Hz, 1H), 3.43(td, J=8.8, 3.2Hz, 1H), 3.20(dd, J=16.4 ,8.7Hz,1H),3.08(dd,J=15.8,0.8Hz,1H),2.51(d,J=15.8Hz,1H),1.98–1.84(m,2H),1.79(dtd,J=10.0, 6.4,3.2Hz,1H),1.29–1.23(m,11H),1.13(s,10H); 13 C NMR (126MHz, CDCl 3 )δ186.7, 149.4, 148.5, 144.5, 143.7, 138.7, 130.2, 121.3, 118.5, 117.5, 113.1, 77.3, 77.0, 76.8, 64.1, 47.5, 39.5, 38.0, 35.0, 34.9, 29.5, 29.5, 27 Resolution mass spectrometry (ESI): calcd.forC 25 h 32 BrNO[M+H] + :442.1667, actual value: 442.1677.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com