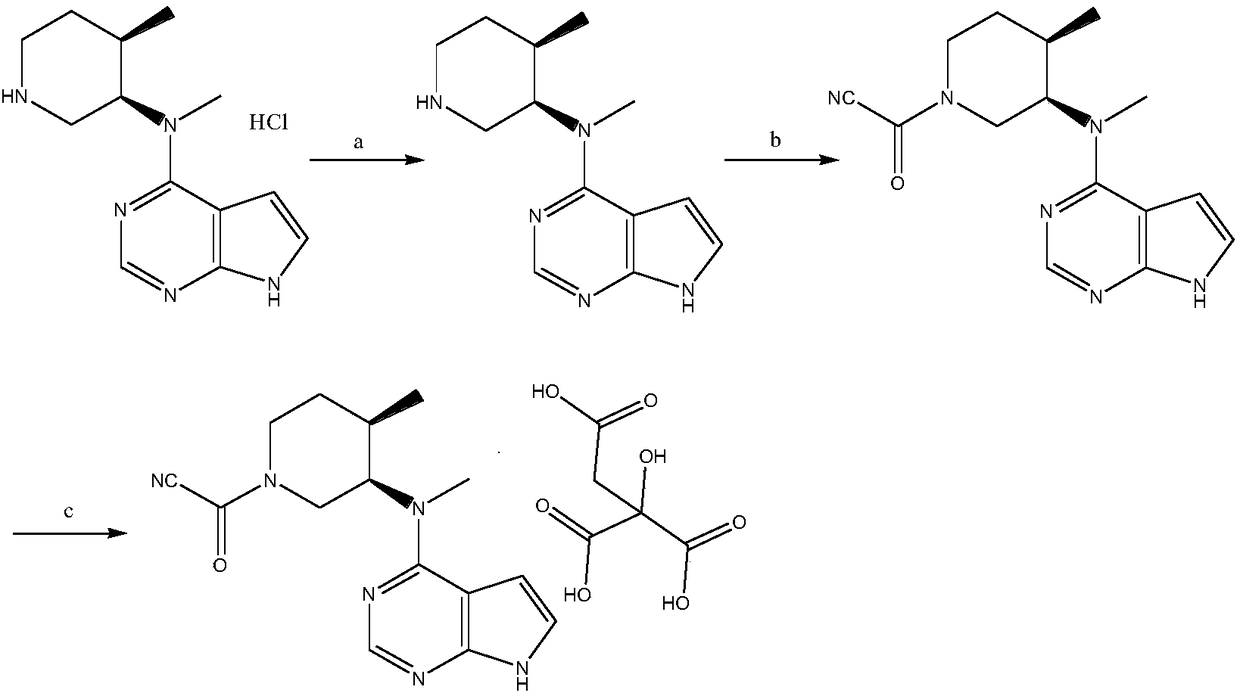
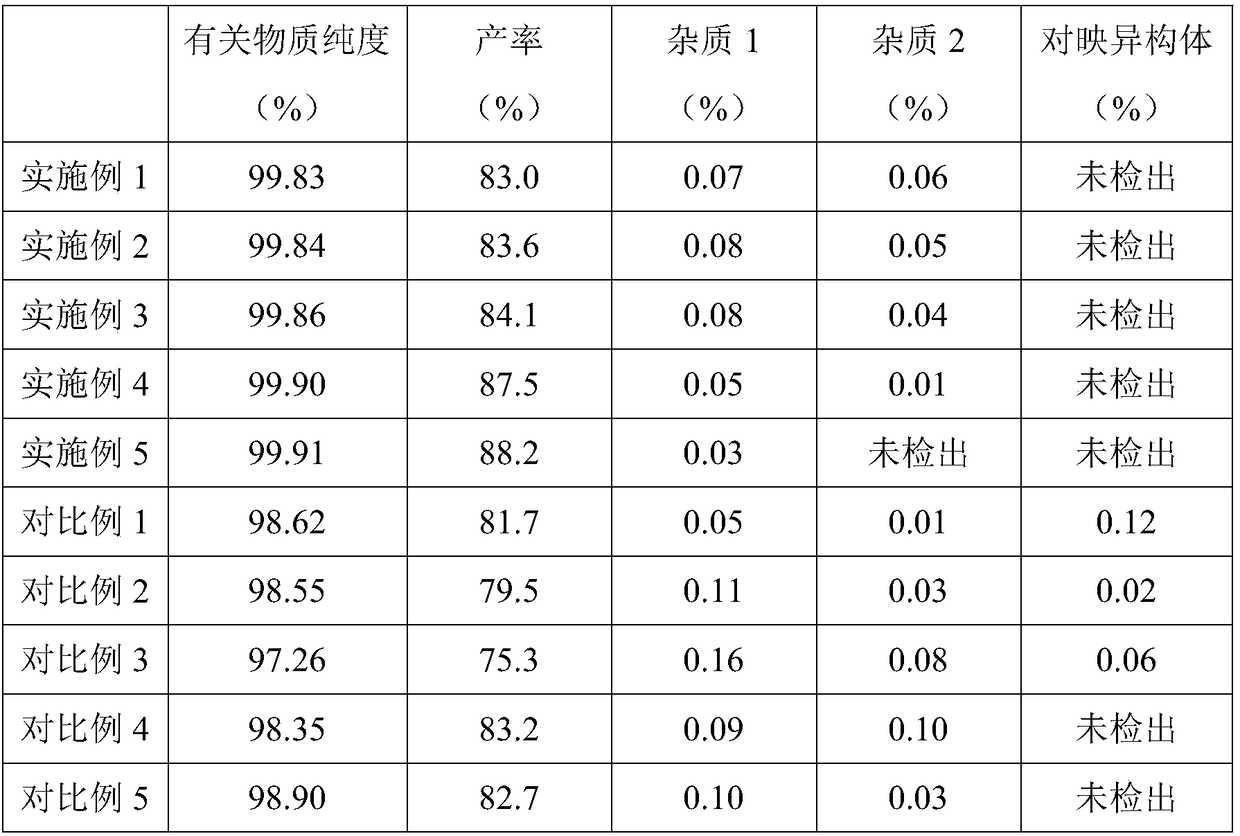
Method for preparing Tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of medicine, can solve the problems of forming many by-products, unfavorable to industrialized production, hindering domestic industrialized production of tofacitinib citrate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In order to solve the above problems, the present invention provides a preparation method of tofacitinib citrate, comprising the following steps:
[0030] a. N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine hydrochloride was added to the In a mixed solvent of methyl sulfoxide and purified water, react in the presence of alkali metal hydroxide to obtain N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl] -7H-pyrrolo[2,3-d]pyrimidin-4-amine;
[0031] b. N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine with cyanoacetylating reagent in catalyst Under the catalysis of , the reaction is carried out in a solvent, after the reaction is completed, a weakly basic inorganic salt solution is added and stirred for 8 to 12 hours to precipitate, filter and dry to obtain a crude product of tofacitinib; the catalyst is 1-(3-dimethylformaldehyde) A mixture of aminopropyl)-3-ethylcarbodiimide hydrochloride, 1-hydroxybenzotriazole and N...
Embodiment 1
[0067] Embodiment 1 provides a kind of preparation method of tofacitinib citrate, comprising the following steps:
[0068] a. Add 10kg of N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidine-4-amine salt to the reactor acid salt, 30kg dimethyl sulfoxide, 20L purified water, 1.5kg sodium hydroxide, heat up to 70°C; after the reaction is completed, stir and crystallize for 2h, suction filtration, and dry to obtain product a; the obtained product a is used for the reaction In the kettle, add 50kg of absolute ethanol, heat to 60°C until the product a is completely dissolved, add 0.5kg of medicinal charcoal for decolorization for 15min; filter while hot, collect the filtrate, cool to 10°C, stir and crystallize for 2h, suction filtration, dry, to obtain the N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine;
[0069] b. add N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine to the reactor, 20L dichlorometha...
Embodiment 2
[0072] Embodiment 2 provides a kind of preparation method of tofacitinib citrate, comprising the following steps:
[0073] a. Add 10kg of N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidine-4-amine salt to the reactor acid salt, 50kg of dimethyl sulfoxide, 30kg of purified water, 2kg of sodium hydroxide, and the temperature was raised to 70 ° C; after the reaction was completed, stirring and crystallization for 2h, suction filtration, and drying to obtain product a; the obtained product a was as the reaction kettle Add 50kg of absolute ethanol, heat to 60°C until the product a is completely dissolved, add 0.5kg of medicinal charcoal for decolorization for 15min; filter while hot, collect the filtrate, cool down to 10°C, stir and crystallize for 2h, suction filtration, and dry to obtain The N-methyl-N-[(3R,4R)-4-methylpiperidin-3-yl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine;
[0074] b. add N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com