A large-area method for preparing self-supporting high-performance oxygen evolution electrodes at room temperature
An oxygen evolution electrode and large-area technology, which is applied in the field of large-area preparation of self-supporting high-performance oxygen evolution electrodes, can solve the problems of inability to achieve large-area growth, consumption of large energy and waste liquid, and difficult control of growth conditions, and achieve excellent oxygen evolution. Oxygen activity and stability, energy cost saving, low production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Disperse the commercial oxide powder (magnesia) in a mixed solvent of ethanol and NMP with a volume ratio of 1:9, and ultrasonically disperse to obtain a 5 mg / mL suspension.
[0029] (2) The suspension solution prepared in step 1 is drip-coated on the surface of the nickel foam substrate, and the coating amount is 0.5mg / cm 2 , and then dried at 60°C.
[0030] (3) Immerse the composite electrode prepared in step 2 into the solution of nickel nitrate and ferrous sulfate, the concentration of metal ions is 0.05M, the molar ratio of Ni / Fe is 8:2, and the reaction time is 0.5 hours.
[0031] (4) The electrode in step 3 was taken out, washed and dried to obtain the target oxygen evolution electrode, which was directly used for OER test.
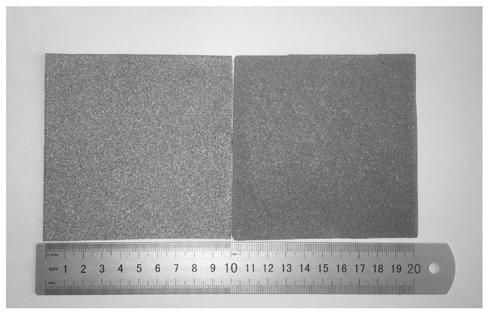
[0032] pass Figure 1a Digital photos can clearly see that the area is 10*10cm 2 A brown-yellow substance grows on the surface of the blank nickel foam (the color on the left is light) (the color on the right becomes darker).
[0033]...
Embodiment 2
[0036] (1) Disperse commercial oxide powder (alumina) in terpineol solvent, and ultrasonically disperse to obtain a 5 mg / mL suspension.
[0037] (2) Drop-coat the suspension solution prepared in step 1 on the surface of conductive PET, and the coating amount is 0.1mg / cm 2 , and then dried at 60°C.
[0038] (3) Immerse the composite electrode prepared in step 2 into nickel nitrate and cobalt nitrate solution, the metal ion concentration is 0.05M, the Ni / Co molar ratio is 8:2, and the reaction time is 0.5 hours.
[0039] (4) The electrode in step 3 was taken out, washed and dried to obtain the target oxygen evolution electrode, which was directly used for OER test.
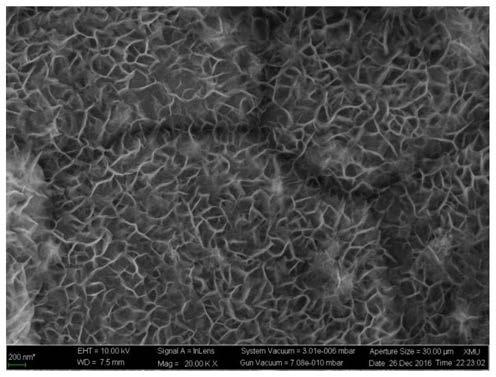
[0040] Depend on figure 2 It can be seen that under this growth condition, sheet-like hydroxides can also grow on the electrode surface.
Embodiment 3
[0042] (1) Disperse commercial oxide powder (calcium oxide) in ethyl acetate solvent, and ultrasonically disperse to obtain a 5 mg / mL suspension.
[0043] (2) Drop-coat the suspension solution prepared in step 1 on the surface of the conductive glass substrate, and the coating amount is 3mg / cm 2 , and then dried at 60°C.
[0044](3) Immerse the composite electrode prepared in step 2 in the solution of nickel nitrate and ferrous sulfate, the concentration of metal ions is 0.05M, the molar ratio of Ni / Fe is 8:1, and the reaction time is 0.5 hours.
[0045] (4) The electrode in step 3 was taken out, washed and dried to obtain the target oxygen evolution electrode, which was directly used for OER test.
[0046] Depend on image 3 It can be seen that under this growth condition, sheet-like hydroxides can also grow on the surface of the electrode, and the sheets are thicker due to the higher loading capacity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com