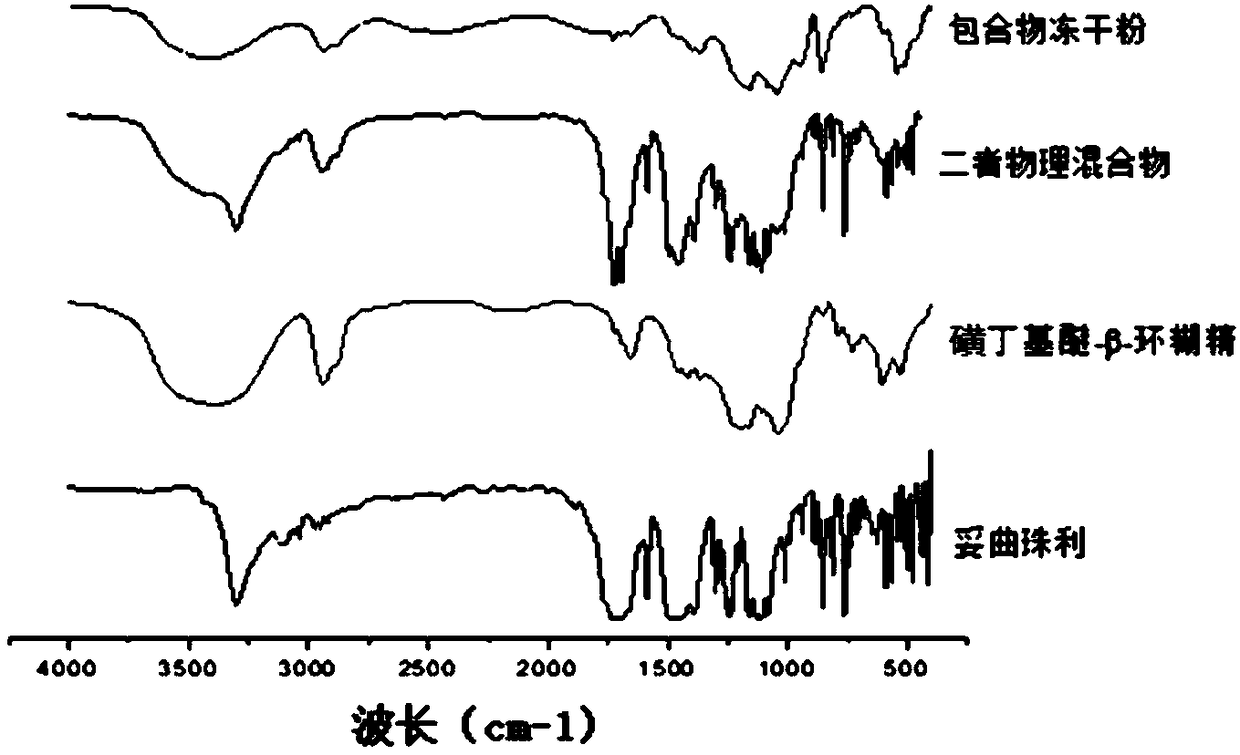
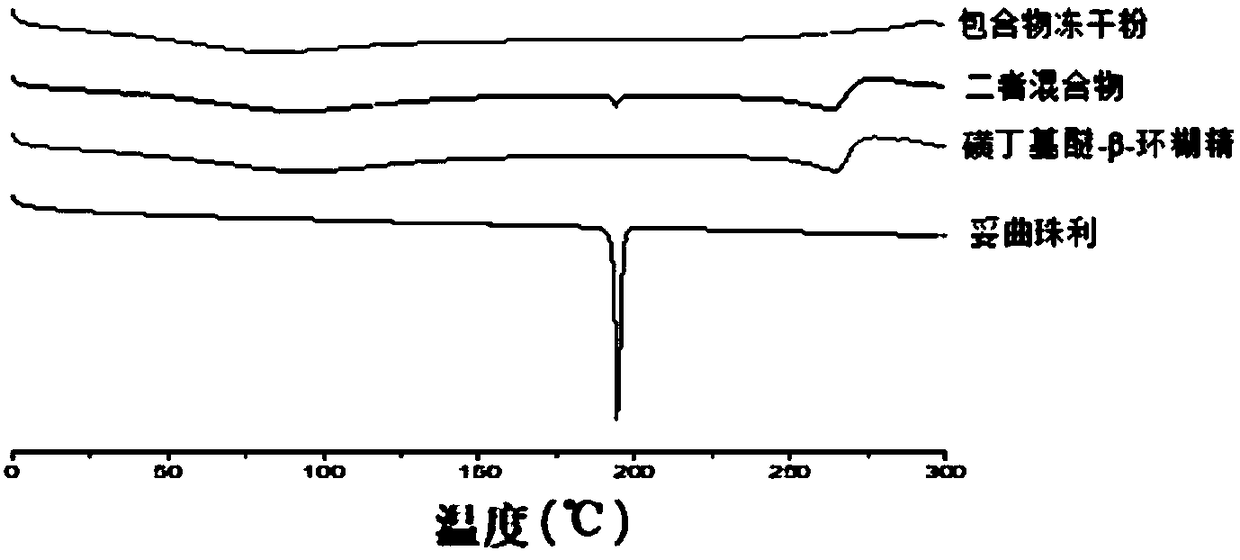
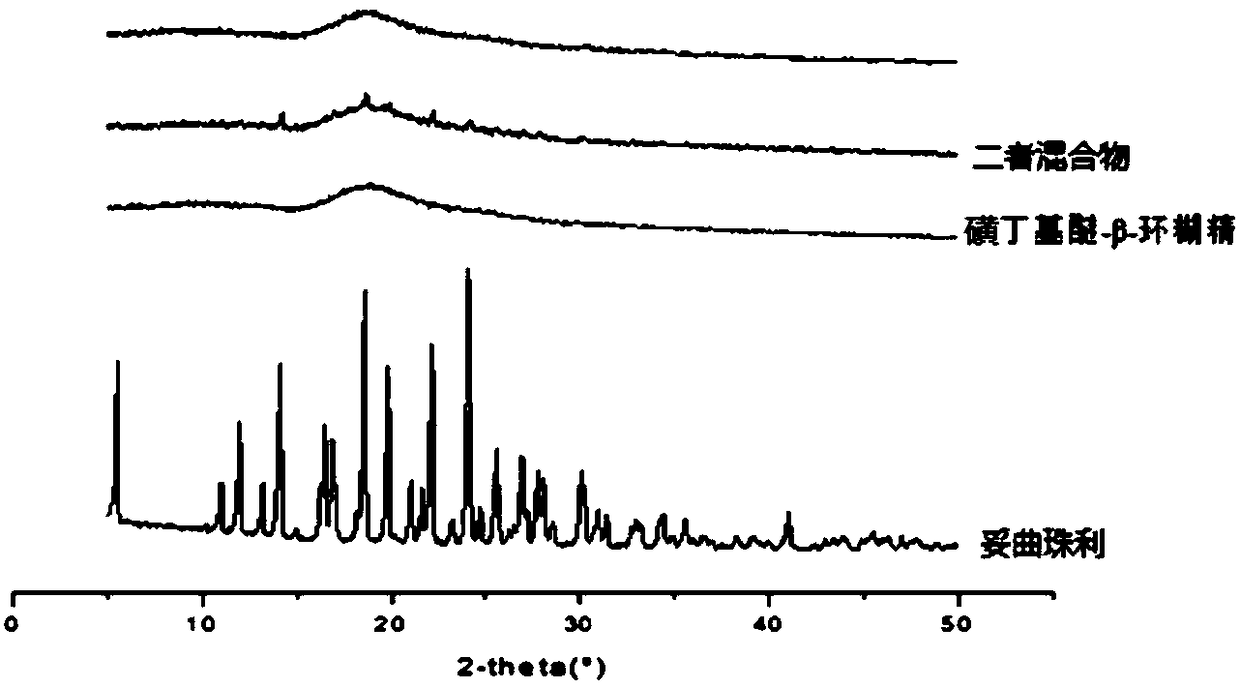
Toltrazuril inclusion compound lyophilized powder and preparation method thereof
A technology of toltrazuril and inclusion complex, applied in the field of toltrazuril inclusion complex freeze-dried powder and preparation thereof, can solve the problems of poor stability, poor water solubility of toltrazuril, limited therapeutic effect, etc. Solubility, the effect of enriching the dosage form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Step 1, dissolving Toltrazuril in an organic solvent to obtain an organic solution of Toltrazuril; the organic solvent is methanol, and the volume concentration is 30%;
[0093] In step 2, the organic solution of toltrazuril obtained in step 1 is added to the aqueous solution of sulfobutyl ether-β-cyclodextrin, and the mass ratio of toltrazuril to sulfobutyl ether-β-cyclodextrin is 2: 1. Magnetic stirring reaction at 300r / min and 60°C for 4h;
[0094] In step 3, the substance reacted in step 2 is rotated to evaporate the organic solvent, then filtered through a 0.45 μm microporous membrane, and dried to obtain the inclusion compound of toltrazuril;
[0095] Step 4: Pre-freeze the toltrazuril inclusion compound obtained in step 3 to -35°C for 3 hours, then freeze at -75°C for 10 hours, freeze-dry at -50°C for 24 hours, and finally heat up to 30°C for drying After 5 hours, the lyophilized powder of Toltrazuril inclusion compound was obtained.
Embodiment 2
[0097] Step 1, dissolving Toltrazuril in an organic solvent to obtain an organic solution of Toltrazuril; the organic solvent is ethanol, and the volume concentration is 30%;
[0098] In step 2, the organic solution of toltrazuril obtained in step 1 is added to the aqueous solution of sulfobutyl ether-β-cyclodextrin, and the mass ratio of toltrazuril to sulfobutyl ether-β-cyclodextrin is 3: 1. Magnetic stirring reaction at 350r / min and 50°C for 5h;
[0099] In step 3, the substance reacted in step 2 is rotated to evaporate the organic solvent, then filtered through a 0.45 μm microporous membrane, and dried to obtain the inclusion compound of toltrazuril;
[0100] Step 4: Pre-freeze the toltrazuril inclusion compound obtained in step 3 to -40°C for 5 hours, then freeze at -80°C for 12 hours, freeze-dry at -55°C for 22 hours, and finally raise the temperature to 35°C for drying After 4 hours, the lyophilized powder of Toltrazuril inclusion compound was obtained.
Embodiment 3
[0102] Step 1, dissolving Toltrazuril in an organic solvent to obtain an organic solution of Toltrazuril; the organic solvent is methanol, and the volume concentration is 40%;
[0103] In step 2, the organic solution of toltrazuril obtained in step 1 is added to the aqueous solution of sulfobutyl ether-β-cyclodextrin, and the mass ratio of toltrazuril to sulfobutyl ether-β-cyclodextrin is 4: 1. Magnetic stirring reaction at 400r / min and 30°C for 6h;
[0104] In step 3, the substance reacted in step 2 is rotated to evaporate the organic solvent, then filtered through a 0.45 μm microporous membrane, and dried to obtain the inclusion compound of toltrazuril;
[0105] Step 4: Pre-freeze the toltrazuril inclusion compound obtained in step 3 to -45°C for 4 hours, then freeze at -85°C for 11 hours, freeze-dry at -52°C for 21 hours, and finally heat up to 32°C for drying After 4.5 hours, the lyophilized powder of Toltrazuril inclusion compound was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com