Perovskite catalyst, preparation method thereof and in-situ testing method of perovskite catalyst
A perovskite and catalyst technology, which is applied in the field of high-performance catalyst materials, can solve problems such as poor reproducibility and uneven distribution of active components, and achieve good efficiency, excellent anti-carbon deposition, and improved anti-coking performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
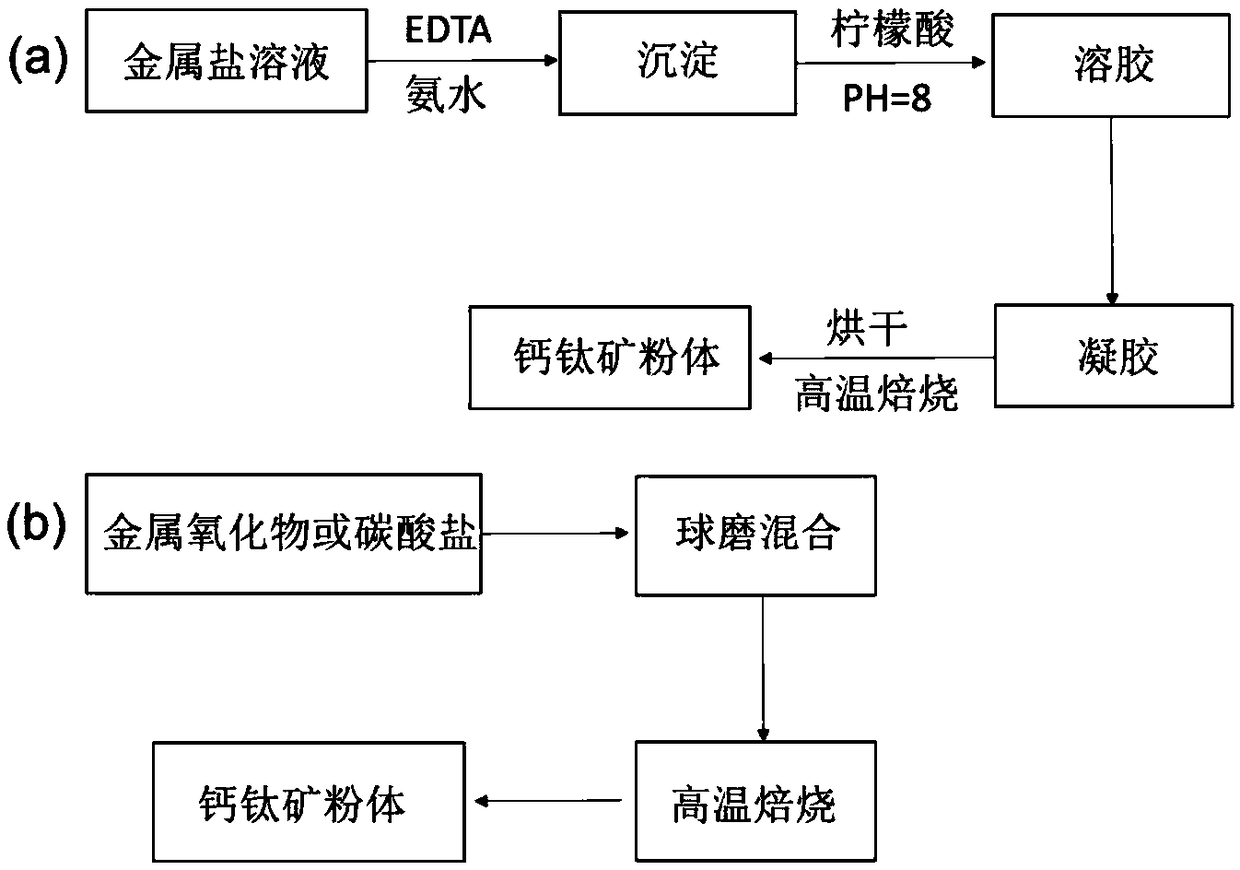
[0055] Implementation Example 1, Ni-doped LaMnO 3 Based perovskite materials
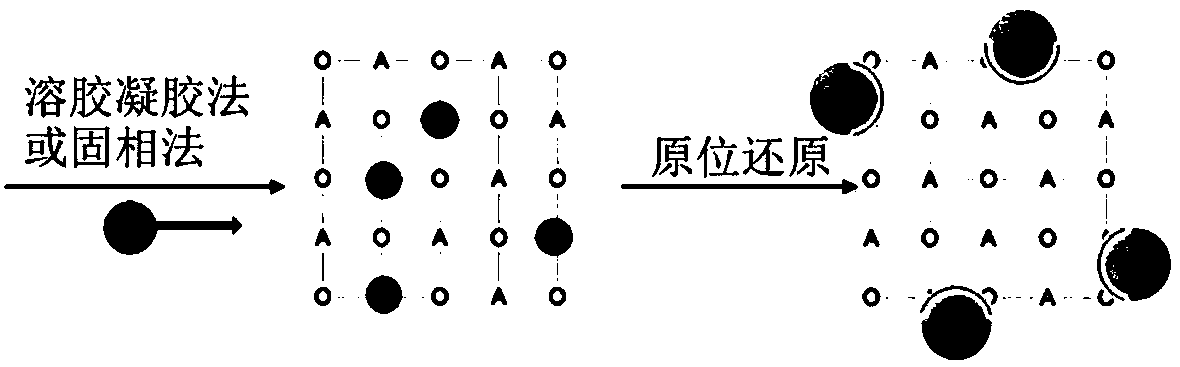
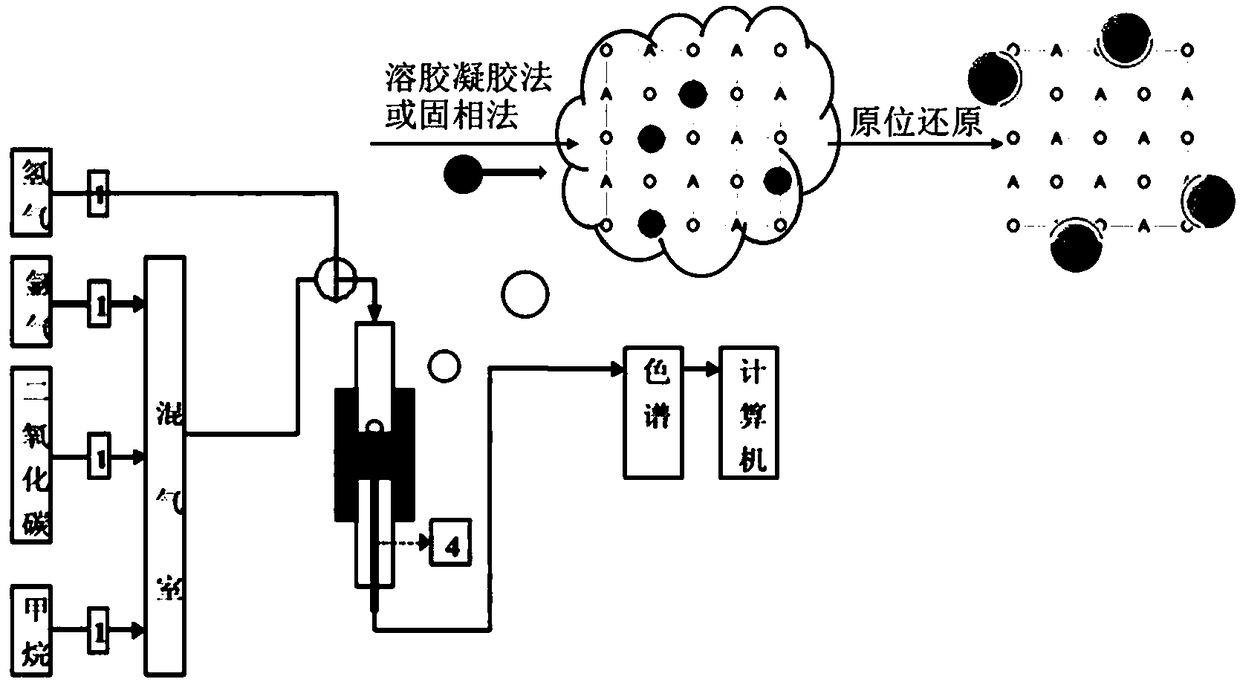
[0056] Some studies have shown that the A-site vacancy of perovskite can provide a driving force to precipitate B-site elements so that the local changes back to ABO 3 ideal structure. La 0.9 mn 0.8 Ni 0.2 o 3 Precursor powder. In order to observe the effect after reduction alone, 800 degrees 5% H 2 -N 2 After 6 hours of reduction, the powder was taken out from the quartz tube. Through scanning electron microscopy, it was found that Ni particles of about 20 nm were evenly dispersed on the surface of the perovskite particles. The distribution of Ni particles was significantly more uniform than that of ordinary supported catalysts, which can be further confirmed by TEM. Precipitation of Ni particles and strong adhesion. After confirming the good precipitation effect, the perovskite precursor was pretreated in the self-made reactor according to the same reduction conditions, and then switched ...
Embodiment 2
[0058] Implementation Example 2, Ni-doped LaSrCrO 3 Based perovskite materials
[0059] Synthesis of La by sol-gel method according to stoichiometric ratio 0.8 Sr 0.1 Cr 0.85 Ni 0.15 o 3 Type A vacant perovskite materials are fired at a high temperature of 1300 degrees to form phases. 800 degrees 5% H 2 -N 2 A large number of Ni particles were uniformly dispersed on the surface of the substrate after being reduced under the atmosphere for 4 hours, and the X-ray diffraction analysis detected that the substrate still maintained the perovskite structure, indicating that this type of perovskite material has good thermal stability. Due to the precipitation of a large amount of metal Ni, at 800 degrees 5%H 2 -N 2 The in situ precipitation-type perovskite materials in the atmosphere have good electrical conductivity, and these advantages can make this Ni-precipitation-type perovskite materials have a wide range of applicability.
Embodiment 3
[0060] Implementation Example 3, Ni-doped LaTiO 3 Based perovskite materials
[0061] By solid-phase method, according to the stoichiometric ratio La 0.6 Ca 0.2 Ni 0.1 Ti 0.9 o 3 Weigh La 2 o 3 , CaCO 3 ,TiO 2 , Ni(NO 3 ) 2 ·6H 2 After ball milling and mixing, press into blocks and bake at a high temperature of 1400 degrees for 12 hours to form a phase. Powder at 900 degrees 5% H 2 -N 2 After 4 hours of reduction pretreatment in the atmosphere, a large number of Ni particles were uniformly dispersed on the surface of the substrate, and the X-ray diffraction analysis detected that the substrate still maintained the perovskite structure, indicating that this type of perovskite material has good thermal stability.
[0062] The above examples illustrate that the present invention covers the surface of the catalyst substrate by in-situ precipitation of nanoparticles used for catalysis, provides good catalytic performance, avoids carbon deposition in the reforming reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com