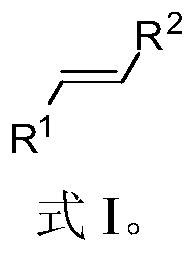
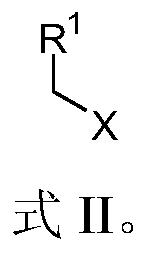A light-induced one-pot method for synthesizing olefinic compounds
A synthesis method and technology for aldehyde compounds, which are applied in the field of light-induced one-pot synthesis of olefin compounds, can solve the problems of hindering the synthesis of functional molecules, limiting the tolerance of functional groups, and inapplicability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
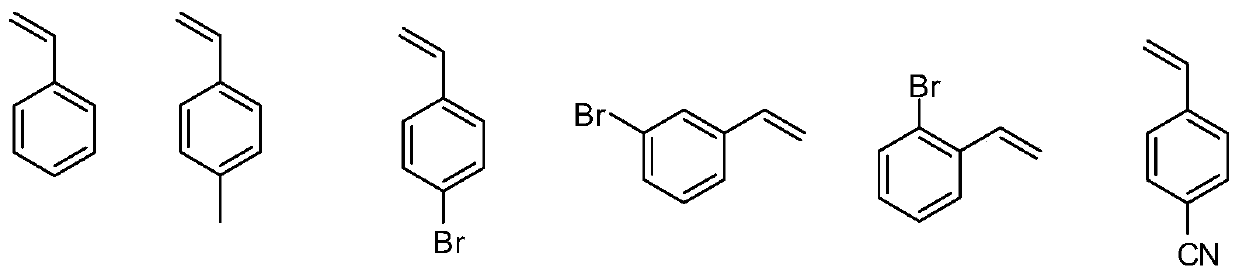
[0051] Add 163.2 mg K to a Schlenk test tube equipped with a magnetic stir bar 2 CO 3 (1.5:1 molar ratio to benzyl chloride), 314.4mg triphenylphosphine (1.5:1 molar ratio to benzyl chloride), 3.8mg Ru(bpy) 3 Cl 2 -6H 2 O (the molar ratio of benzyl chloride to benzyl chloride is 0.005:1), 60mg paraformaldehyde, 10mL acetonitrile, 119μL benzyl bromide or 115μL benzyl chloride, argon blowing for 5 minutes, under visible light, normal temperature, the reaction time is respectively After 4h and 8h, the product was separated and purified by petroleum ether to obtain 70 mg and 62.4 mg of styrene (Formula 1), with yields of 67% and 60%, respectively.
[0052] Styrene: 1 H NMR (CDCl 3 ,400MHz)δ7.41(d,J=6.87Hz,2H),7.32(t,J=6.87Hz,2H),7.24(t,J=6.87Hz,1H),6.72(dd,J 1 =17.40Hz,J 2 =10.99Hz,1H),5.75(d,J=18.78Hz,1H),5.24(d,J=10.99Hz,1H). 13 C NMR (CDCl 3 ,100MHz)δ137.7,137.0,128.6,127.9,126.3,113.9.EI-MS:M + m / z 104.
Embodiment 2
[0054]
[0055] Add 163.2 mg K to a Schlenk test tube equipped with a magnetic stir bar 2 CO3 (1.5:1 molar ratio to 4-methylbenzyl bromide), 314.4mg triphenylphosphine (1.5:1 molar ratio to 4-methylbenzyl bromide), 3.8mg Ru(bpy) 3 Cl 2 -6H 2 O (molar ratio to 4-methylbenzyl bromide is 0.005:1), 60mg paraformaldehyde, 10mL acetonitrile, 185mg 4-methylbenzyl bromide, argon blowing for 5 minutes, under visible light, normal temperature, reaction time After 4 hours, the product was separated and purified by petroleum ether to obtain 90 mg of 4-methylstyrene (Formula 2), with a yield of 76%.
[0056] 4-Methylstyrene: 1 H NMR (CDCl 3 ,400MHz)δ7.33(d,J=8.24Hz,2H),7.16(d,J=7.79Hz,2H),6.72(dd,J 1 =17.40Hz,J 2 =10.99Hz,1H),5.72(d,J=17.40Hz,1H),5.21(d,J=10.99Hz,1H),2.37(s,3H). 13 C NMR (CDCl 3 ,100MHz)δ137.7,136.8,135.0,129.3,126.3,112.9,21.3.EI-MS:M + m / z 118.
Embodiment 3
[0058]
[0059] Add 163.2 mg K to a Schlenk test tube equipped with a magnetic stir bar 2 CO 3 (The molar ratio to p-bromobenzyl bromide is 1.5:1), 314.4mg triphenylphosphine (the molar ratio to p-bromobenzyl bromide is 1.5:1), 3.8mg Ru(bpy) 3 Cl 2 -6H 2 O (the molar ratio to p-bromobenzyl bromide is 0.005:1), 60mg of paraformaldehyde, 10mL of acetonitrile, 248mg of 4-bromobenzyl bromide, bubbling with argon for 5 minutes, under visible light, at room temperature, the reaction time is 4h, the product after Petroleum ether was separated and purified to obtain 164 mg of 4-bromostyrene (Formula 3) with a yield of 93%.
[0060] 4-Bromostyrene: 1H NMR (CDCl 3 ,400MHz)δ7.43(d,J=8.70Hz,2H),7.26(d,J=8.70Hz,2H),6.64(dd,J 1 =17.63Hz,J 2 =10.99Hz,1H),5.73(d,J=17.40Hz,1H),5.27(d,J=10.99Hz,1H).13C NMR(CDCl3,100MHz)δ136.6,135.9,131.7,127.9,121.7,114.7. EI-MS: M+m / z 182.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com