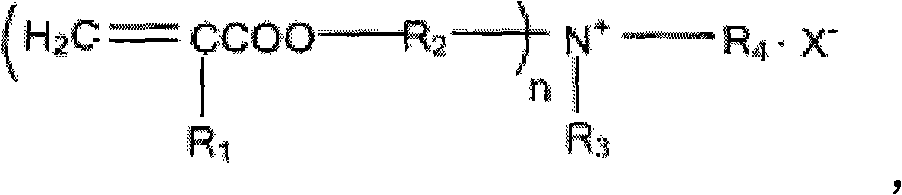
Cross-linked polyquaternary ammonium salt type antibiosis monomer, preparation method of cross-linked polyquaternary ammonium salt type antibiosis monomer and application of cross-linked polyquaternary ammonium salt type antibiosis monomer in dentistry repairing materials
A cross-linking polymerization, quaternary ammonium salt technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, dentistry, etc., can solve the problems of no bonding cross-linking system, decreased mechanical properties of materials, and inappropriate use of light-colored materials. , to reduce the probability that the double bond does not participate in the polymerization reaction, improve the safety of use, and achieve the effects of simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
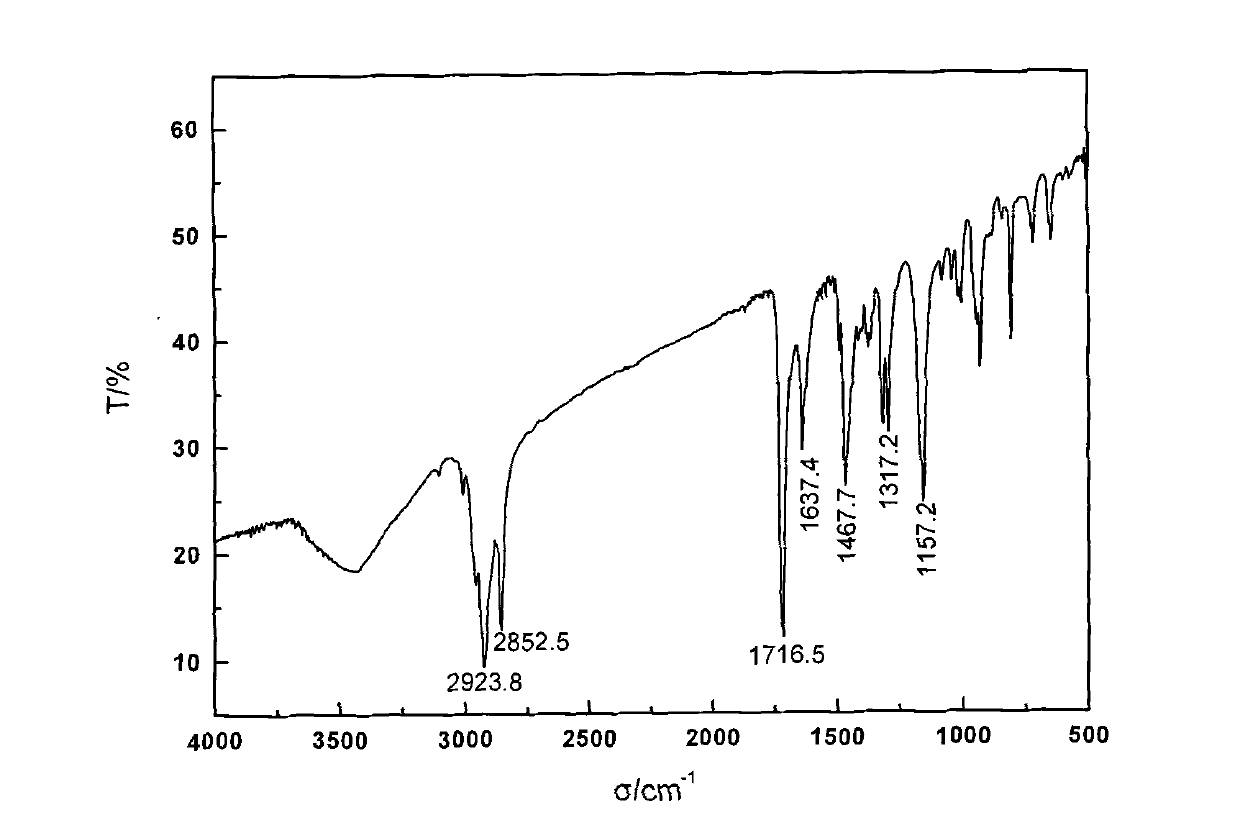
[0043] The steps of the preparation method of the crosslinkable polymerized quaternary ammonium salt type antibacterial monomer of the present invention are as follows:
[0044] In the first step, in the tertiary amine substance with OH group, any one of acetone, dichloromethane or acetonitrile is added as a solvent, and the halogenated hydrocarbon substance is added for reflux reaction to obtain product A, which is The quaternary ammonium salt of the hydroxyl group is added with a molar ratio of tertiary amine:halogenated hydrocarbon=1:1, and the reaction time is 6-36h. The tertiary amine has two or three OH groups, which are N-methyldiethanolamine, N-ethyl Diethanolamine or triethanolamine, halogenated hydrocarbons are 1-bromododecane, 1-bromohexadecane or benzyl chloride;
[0045] In the second step, the product A prepared in the first step reacts with any one of toluene, benzene, methylene chloride or tetrahydrofuran as a solvent, and adds an unsaturated acid chloride cont...
Embodiment 1
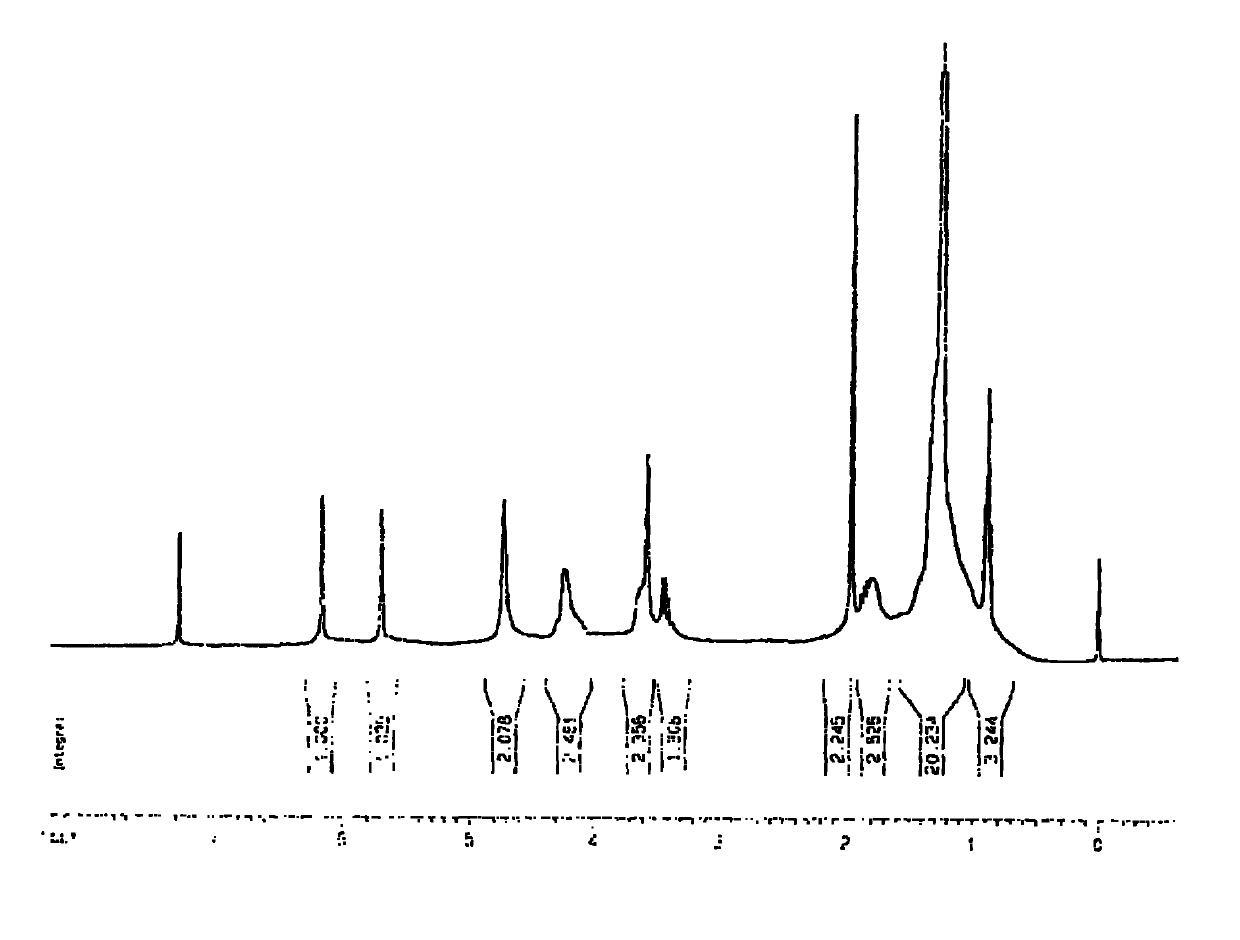
[0053] In the first step, add 0.1mol N-methyldiethanolamine to 100mL of acetonitrile, then add 1-bromohexadecane for reflux reaction, the molar ratio of the two is 1:1, and the reaction time is 20h. Dry to obtain white powdery product A;
[0054] In the second step, add 100 mL of dichloromethane to 0.05 mol of the above product A as a solvent, then add methacryloyl chloride to reflux reaction, the molar ratio of feed is A:acyl chloride=1:2.2, and react for 6 hours. Crystallization, vacuum drying and other methods to obtain the product 2-(methacryloyloxyethyl)-n-hexadecyl-methyl ammonium bromide;
[0055] In the third step, 2-(methacryloyloxyethyl)-n-hexadecyl-methylammonium bromide is mixed in the order of 0%, 1%, 2%, 3%, 4%, 5%, 6%, Add 7%, 8%, 9%, 10%, 15% (w / w) into a certain amount of Bis-GMA / HEMA, with N,N-dimethylaminoethyl methacrylate (DMAEMA) as the auxiliary initiator agent, camphorquinone (CQ) as a photoinitiator, ultraviolet light irradiation polymerization to pr...
Embodiment 2
[0063] In the first step, 0.1 mol of N-ethyldiethanolamine was added to 100 mL of acetone, and then 1-bromododecane was added for reflux reaction. The molar ratio of the two was 1:1, and the reaction time was 36 hours. After the reaction, the white powdery product A was obtained by suction filtration and drying;
[0064] In the second step, 100 mL of benzenedichloromethane was added to 0.05 mol of the above-mentioned product A as a solvent, and then acryloyl chloride was added for reflux reaction, and the molar ratio of feeding was A:acyl chloride=1:2.2, and the reaction was carried out for 6 hours. After the reaction, the product 2-(acryloyloxyethyl)-n-dodecyl-ethylammonium bromide was obtained by rotary evaporation, recrystallization, vacuum drying and other methods;
[0065] In the third step, 2-(acryloyloxyethyl)-n-dodecyl-ethylammonium bromide is added at 0%, 1%, 2%, 3%, 4%, 5%, 6%, 7%. , 8%, 9%, 10%, 15% (w / w) added in a certain amount of Bis-GMA / HEMA, with DMAEMA as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com