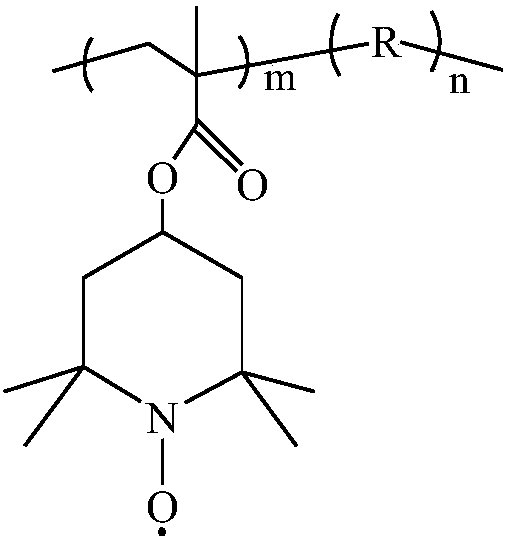
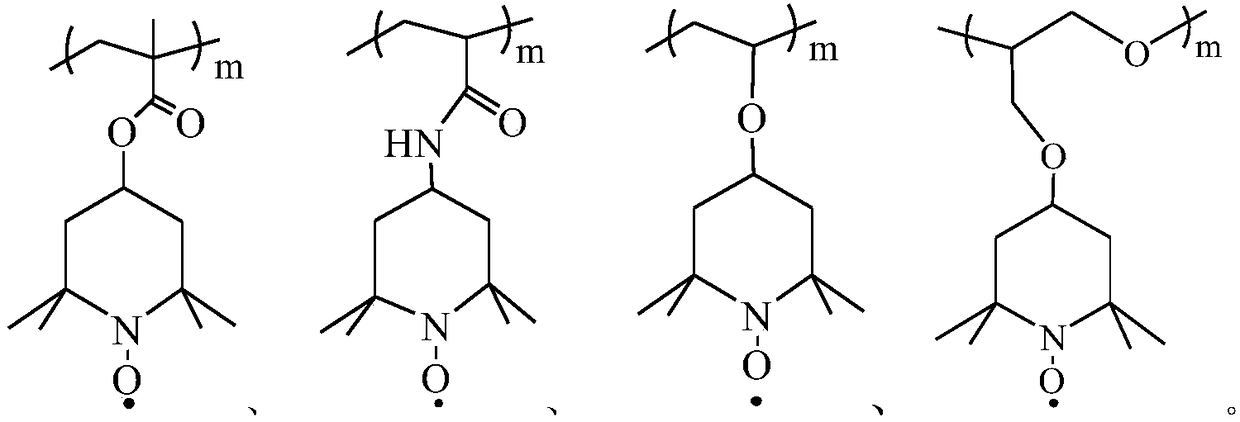
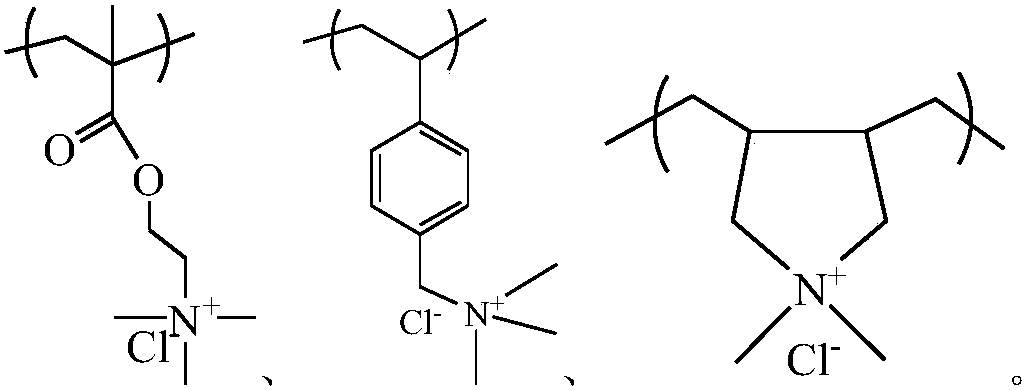
Quaternary ammonium salt-doped nitroxide free radical polymer and preparation method thereof
A technology of nitroxide free radicals and quaternary ammonium salts, which is applied in the field of electrode materials, can solve the problems of low redox capacity, low water solubility, and unstable doping state, etc., and achieve the improvement of charge and discharge speed and cycle performance, fast movement, The effect of increasing the total energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) In the Schlenk reaction tube, mix 4-methacrylic acid-2,2,6,6-tetramethylpiperidinol ester (22.2mmol), methacryloxyethyltrimethylammonium chloride 2 (22.2mmol, 80% aqueous solution), and soluble in dilute hydrochloric acid (0.75mol / L -1 ) In 2-mercaptoethanol (3.5mmol), and then add 4,4'-azobis(4-cyanovaleric acid) (ABCVA) (2.2mmol). The reaction system was kept in an oxygen-free state through three freeze-thaw cycles to cause free radical copolymerization to occur, and the reaction mixture was stirred at 75°C for 24 hours.
[0037] (2) Cool the solution to room temperature, and add hydrogen peroxide (33.3 mmol, 30% aqueous solution), sodium tungstate (0.29 mmol), EDTA (0.1 mmol) and sodium hydroxide aqueous solution (11 ml, 10% by weight). After stirring the solution for 24 hours, the remaining hydrogen peroxide (33.3 mmol) was added. The total reaction time was 48 hours. The solution was dialyzed with water (MWCO=1,000 gmol-1), filtered, and then the product was free...
Embodiment 2
[0039] (1) Combine 4-acrylamido-2,2,6,6-tetramethylpiperidine and methacryloxyethyltrimethylammonium chloride in a molar ratio of 1:1, and 2-mercaptoethanol Dissolve it in dilute hydrochloric acid, add it to the Schlenk reaction tube, and add 4,4'-azobis(4-cyanovaleric acid) (ABCVA). The reaction system was kept in an oxygen-free state through three freeze-thaw cycles to cause free radical copolymerization to occur, and the reaction mixture was stirred at 75°C for 24 hours.
[0040] (2) Cool the solution to room temperature, and add hydrogen peroxide (30% aqueous solution), sodium tungstate, EDTA and sodium hydroxide aqueous solution (Wt=10%). After stirring the solution for 24 hours, the remaining hydrogen peroxide was added. The total reaction time was 48 hours. The solution was dialyzed with water (MWCO=1,000 g mol-1), filtered, and then the product was freeze-dried. The operation was repeated three times to obtain an orange powder.
Embodiment 3
[0042] (1) Combine 4-methacrylic acid-2,2,6,6-tetramethylpiperidinol ester and 4-vinylbenzyltrimethylammonium chloride in a molar ratio of 1:1, and 2-mercapto Ethanol is dissolved in dilute hydrochloric acid and added to the Schlenk reaction tube together, and then 4,4'-azobis(4-cyanovaleric acid) (ABCVA) is added. The reaction system was kept in an oxygen-free state through three freeze-thaw cycles to cause free radical copolymerization to occur, and the reaction mixture was stirred at 75°C for 24 hours.
[0043] (2) Cool the solution to room temperature, and add hydrogen peroxide (30% aqueous solution), sodium tungstate, EDTA and sodium hydroxide aqueous solution (Wt=10%). After stirring the solution for 24 hours, the remaining hydrogen peroxide was added. The total reaction time was 48 hours. The solution was dialyzed with water (MWCO=1,000 g·mol-1), filtered, and then the product was freeze-dried. The operation was repeated three times to obtain an orange powder.
[0044] In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com