Glycyrrhetinic acid prodrug micelle for jointly carrying chemotherapy drug and preparation method thereof
A technology of glycyrrhetic acid and chemotherapeutic drugs is applied in the field of glycyrrhetic acid prodrug micelles and preparation thereof, which can solve the problems of large toxic and side effects, limited application of chemotherapeutic drugs, lack of selectivity in anti-tumor effects, etc. drug resistance, increased entrapment capacity, and enhanced synergistic antitumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
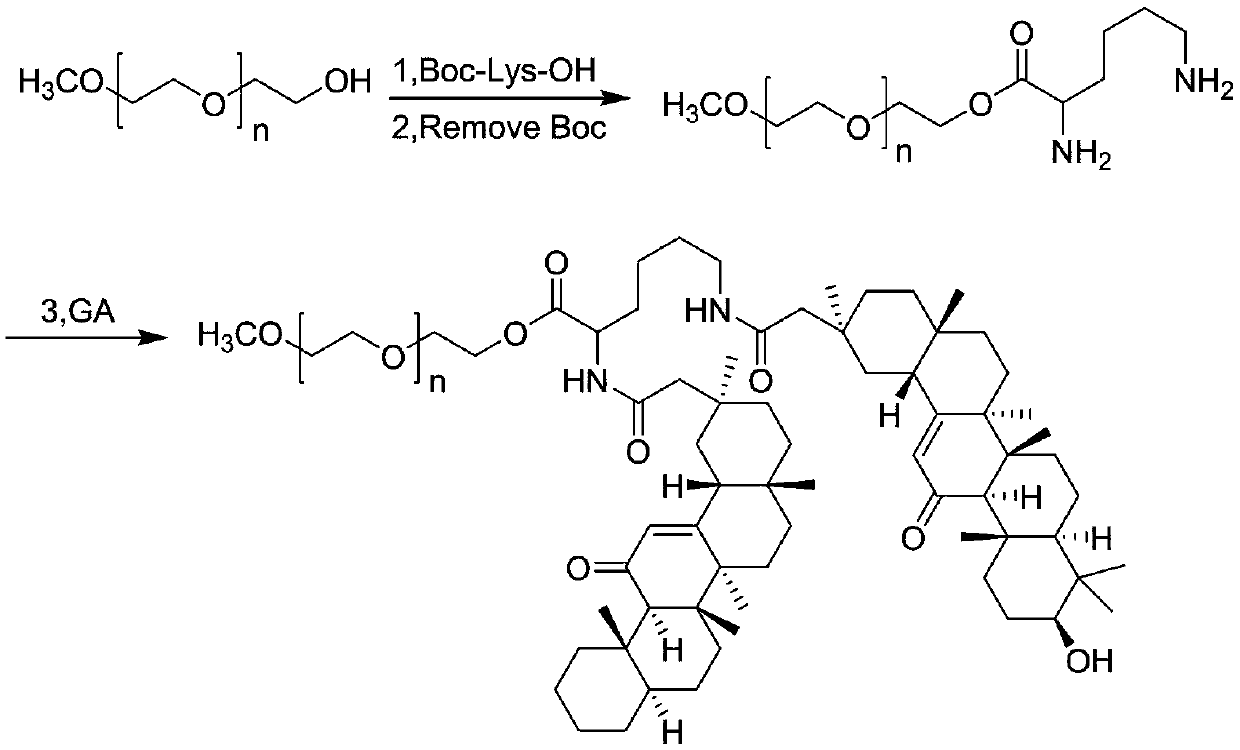
[0035] Embodiment 1: the synthesis of PEG-GA polymer prodrug
[0036] 5 g polyethylene glycol monomethyl ether (molecular weight: 2K), 0.56 g lysine (Boc) 2 , 0.35 g DCC, and 0.01 g DMAP were dissolved in 25 mL of dichloromethane, and reacted with magnetic stirring at room temperature for 1 day, separated and purified by absolute ethanol and ether, and dried under reduced pressure to obtain PEG-lysine as a white solid Acid (Boc) 2 polymer.
[0037] 1 g PEG-Lysine (Boc) 2 The polymer was dissolved in 1.5 mL of dichloromethane and 1.5 mL of trifluoroacetic acid, stirred magnetically at room temperature for 0.5 h, separated and purified by ether, and dried under reduced pressure to obtain a pale yellow oily PEG-lysine polymer de-Boc grouped.
[0038] 1 g of PEG-lysine polymer, 3 g of GA, 0.6 g of DCC and 0.01 g of DMAP were co-dissolved in 10 mL of dichloromethane, stirred at room temperature for 1 day, separated and purified by absolute ethanol and diethyl ether, and reduced ...
Embodiment 2
[0039]Embodiment 2: the synthesis of PEG-GA polymer prodrug
[0040] 5 g polyethylene glycol monomethyl ether (molecular weight: 5K), 1.2 g lysine (Boc) 2 , 0.7 g DCC and 0.04 g DMAP were dissolved in 20 mL of methanol, and reacted with magnetic stirring at room temperature for 3 days, separated and purified by diethyl ether and absolute ethanol, and dried under reduced pressure to obtain a white solid PEG-lysine ( Boc) 2 polymer.
[0041] 1 g PEG-Lysine (Boc) 2 The polymer was dissolved in 5 mL of dichloromethane and 5 mL of trifluoroacetic acid, stirred with magnetic force at room temperature for 2 h, separated and purified by diethyl ether and absolute ethanol, and dried under reduced pressure to obtain a light yellow oily PEG-lysine de-Boc grouped Acid polymers.
[0042] 1 g PEG-lysine polymer, 1.5 g GA, 0.3 g DCC, and 0.02 g DMAP were co-dissolved in 5 mL chloroform and 20 μL triethylamine, and reacted with magnetic stirring at room temperature for 3 days. Separation...
Embodiment 3
[0043] Embodiment 3: the synthesis of PEG-GA polymer prodrug
[0044] 5 g polyethylene glycol monomethyl ether (molecular weight: 2K), 2.5 g lysine (Boc) 2 , 2 g of DCC and 1 g of DMAP were dissolved in 20 mL of dichloromethane and 5 mL of tetrahydrofuran, reacted with magnetic stirring at room temperature for 7 days, separated and purified by petroleum ether, diethyl ether and absolute ethanol, and dried under reduced pressure to obtain White solid PEG-lysine (Boc) 2 polymer.
[0045] 1 g PEG-Lysine (Boc) 2 The polymer was dissolved in 2.5 mL of dichloromethane and 2.5 mL of trifluoroacetic acid, stirred with magnetic force at room temperature for 6 h, separated and purified by petroleum ether and diethyl ether, and dried under reduced pressure to obtain PEG-lysine de-Boc grouped as light yellow oil acid polymer.
[0046] 1 g of PEG-lysine polymer, 2.8 g of GA, 1.3 g of DCC and 0.45 g of DMAP were co-dissolved in 4 mL of dichloromethane and 1 mL of tetrahydrofuran, and 65...
PUM
| Property | Measurement | Unit |
|---|---|---|
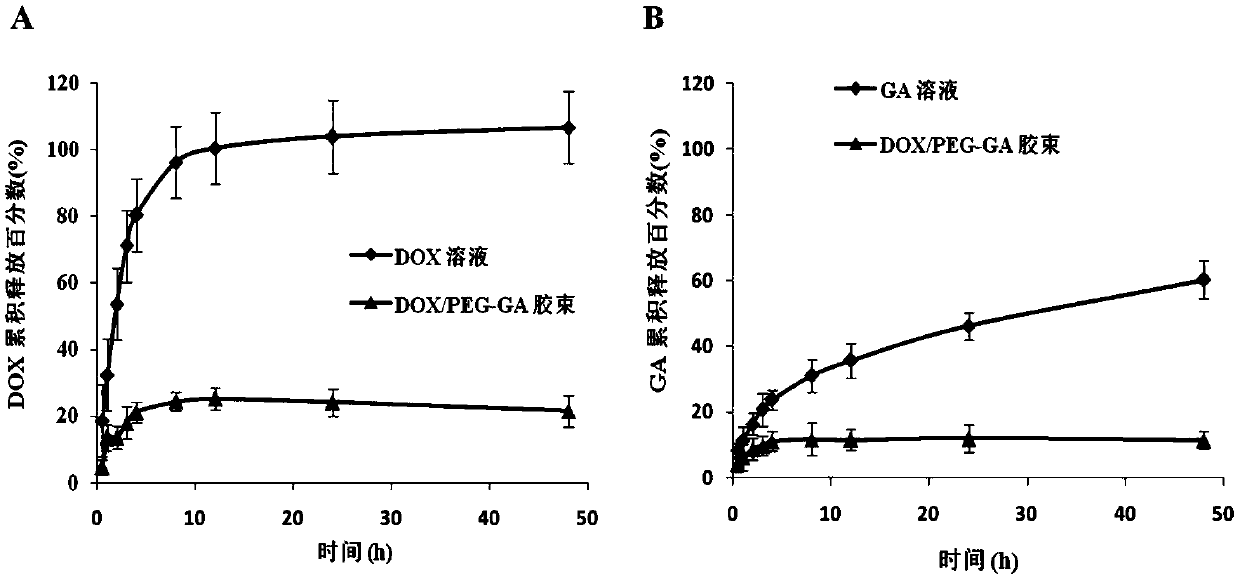
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com