Crystalline methyl carbamate compound
A technology of compounds and compositions, applied in the fields of organic chemistry, organic active ingredients, organic chemistry methods, etc., can solve the problems of patient treatment failure, patients without treatment alternatives, etc., and achieve the effect of low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
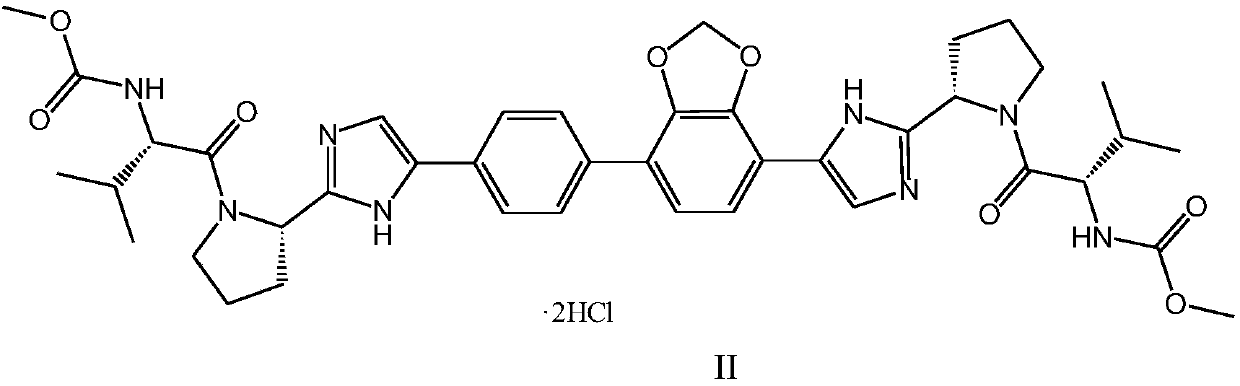
[0041] Embodiment 1 formula (I) compound N-[(2S)-1-[(2S)-2-{4-[7-(4-{2-[(2S)-1-[(2S)-2- [(Methoxycarbonyl)amino]-3-methylbutyryl]pyrrolidin-2-yl]-1H-imidazol-4-yl}phenyl)-2H-1,3-benzodioxol-4 -yl]-1H-imidazol-2-yl}pyrrolidin-1-yl]-3-methyl-1-oxobutane-2-yl]preparation of methyl carbamate (according to the method disclosed in CN102791687B )
[0042]
[0043] step 1
[0044]
[0045] To a solution of ethyl 4-bromo-2,3-dihydroxybenzoate (I-VIh, 1.3 g, 5.0 mmol) in DMF (10.0 mL) was added Cs2CO3 (3.5 g, 11.0 mmol), and the mixture was stirred at room temperature for 1 Hour. To the mixture was added CH2I2 (2.2 g, 8.1 mmol) and the mixture was stirred at 70 °C for 12 hours. The reaction mixture was diluted with ethyl acetate and washed with water and brine. The solvent was removed and the residue was purified by column chromatography on silica gel (eluent: PE:EtOAc=4:1) to provide compound I-IXa (700 mg, yield 52%) as a yellow solid. 1H NMR (400MHz, CDCl3) δ7.31(d, 1H), ...
Embodiment 2
[0064] Embodiment 2 formula (I) compound N-[(2S)-1-[(2S)-2-{4-[7-(4-{2-[(2S)-1-[(2S)-2- [(Methoxycarbonyl)amino]-3-methylbutyryl]pyrrolidin-2-yl]-1H-imidazol-4-yl}phenyl)-2H-1,3-benzodioxol-4 Preparation of -yl]-1H-imidazol-2-yl}pyrrolidin-1-yl]-3-methyl-1-oxobutane-2-yl]carbamate dihydrochloride
[0065] At room temperature, a solution of the pure product of formula II (800 g, 1.0 eq) and ethyl acetate (8 L) was successively added into a 20 L bottle and stirred. Add dropwise HCl / ethyl acetate solution (839g) with a concentration of about 11.2% into the system, control the temperature of the system at 15°C to 25°C, stir for more than 3 hours, stop the reaction, filter with suction, and filter the cake with ethyl acetate (2L) Wash, control the temperature of the filter cake and dry it at 40-60°C, take a sample and test until the residue of ethyl acetate <0.5%, (about 73 hours after drying), the compound of structural formula I is obtained, off-white solid powder or granule, 77...
Embodiment 3
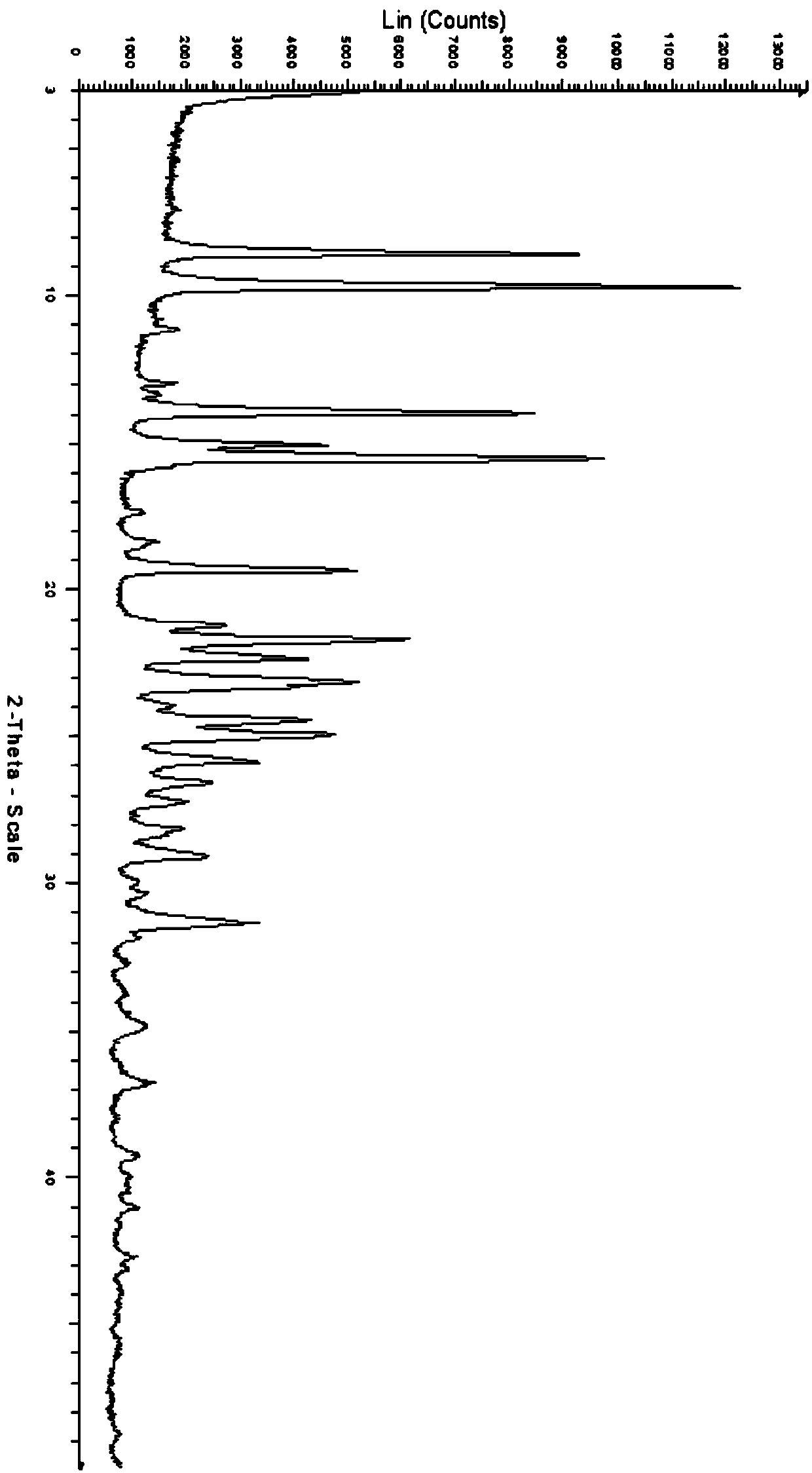
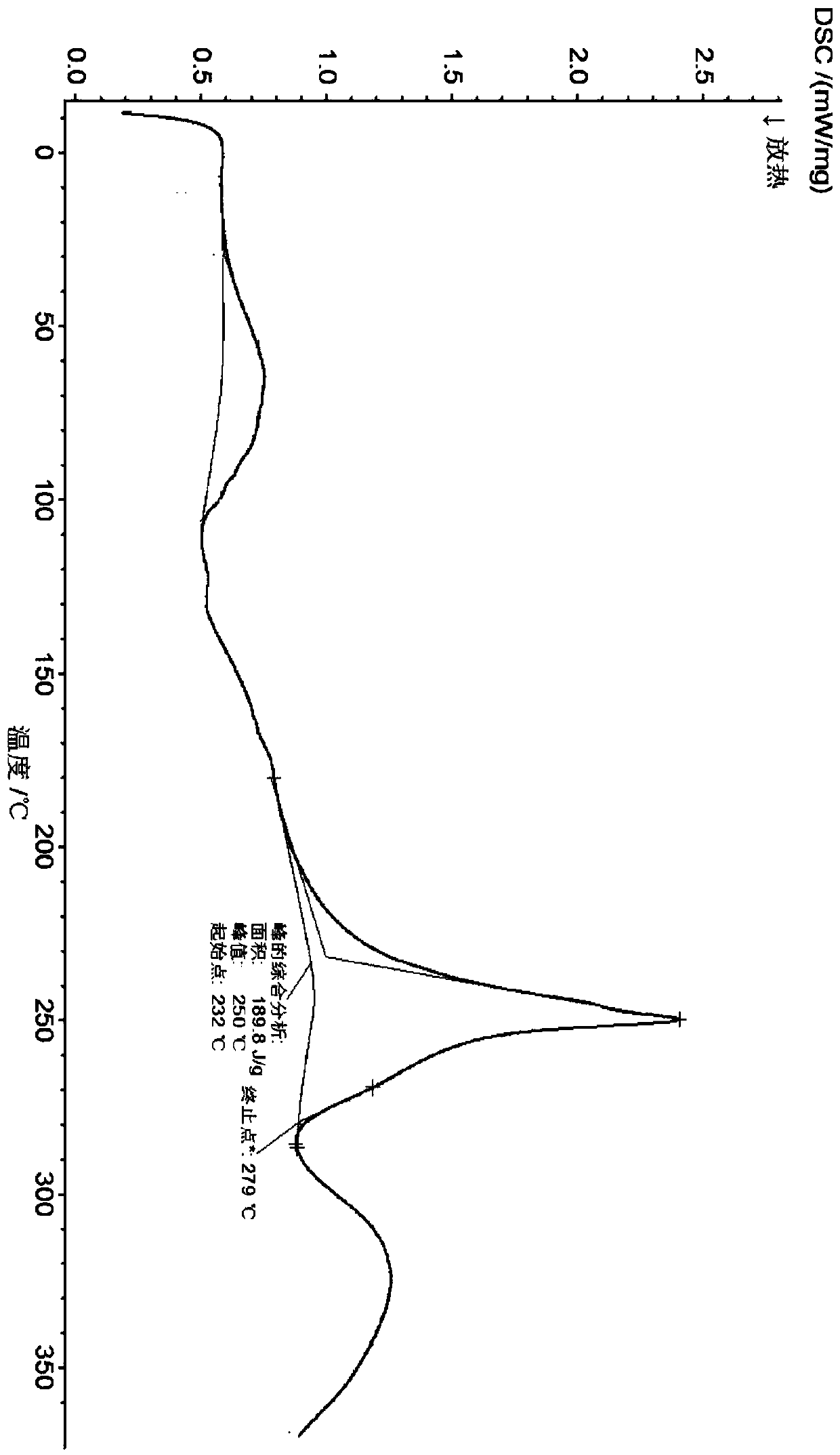
[0066] Embodiment 3 carries out crystal form screening to formula (I) compound with different crystallization methods
[0067] 1. Slow volatile crystallization
[0068] Weigh about 10 mg of the pure product with structural formula I into a 3 mL glass bottle, respectively add about 0.5-1.25 mL of the following solvents to ensure that the samples are completely dissolved to obtain a clear solution, and the obtained solution is slowly volatilized at room temperature. Solid test XRPD, the results are shown in Table 1. The solids obtained in the volatile crystallization experiment were all amorphous, and no other new crystal forms were obtained.
[0069] Experiment number
Solvent used
temperature
get solid
805301-14-A1
H2O
RT
amorphous
805301-14-A2
MeOH
RT
amorphous
805301-14-A3
EtOH
RT
amorphous
805301-14-A4
IPA
RT
N / A*
805301-14-A5
RT
amorphous
805301-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com