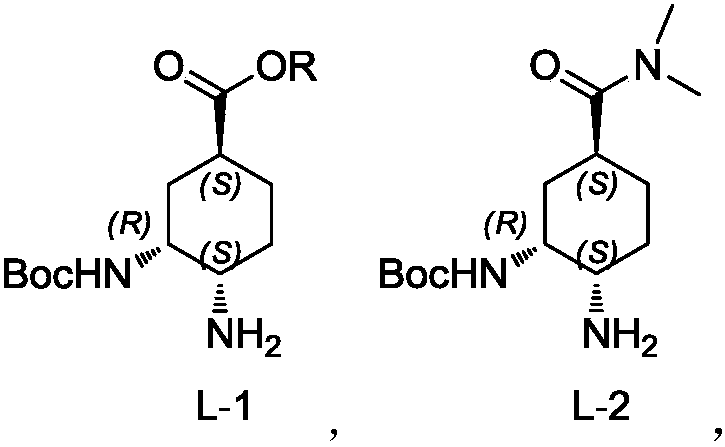
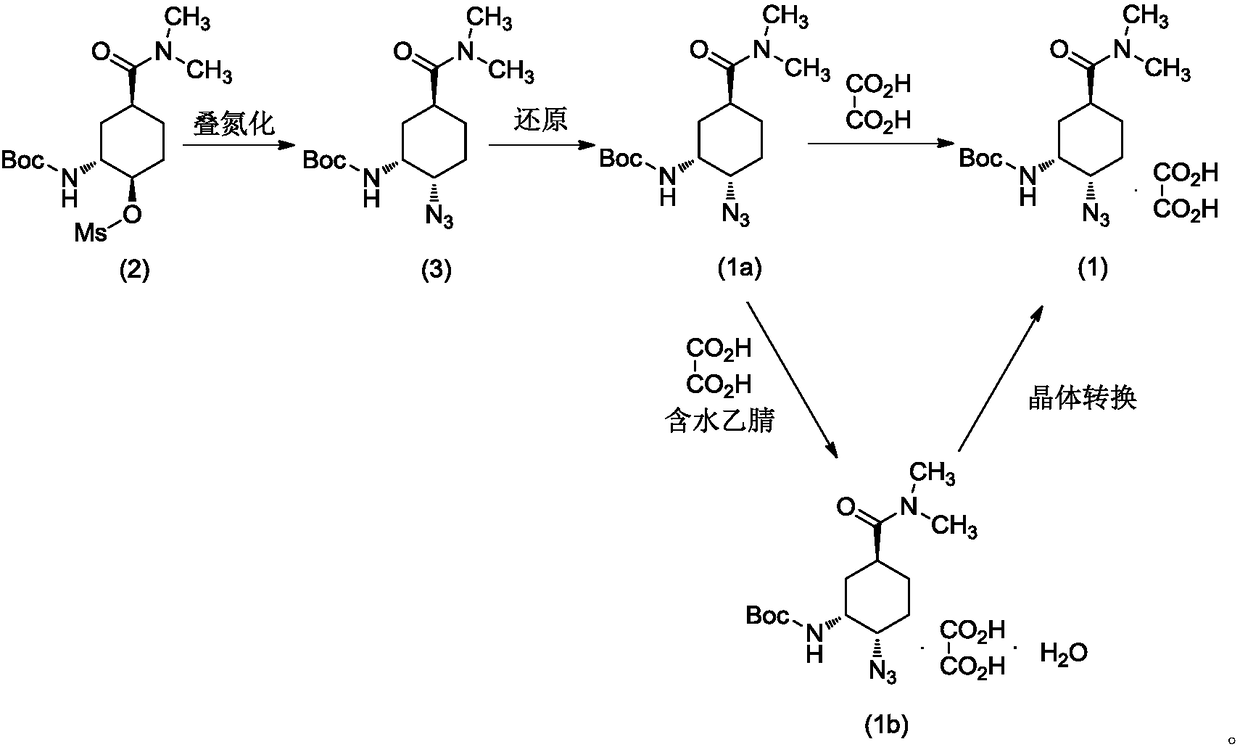
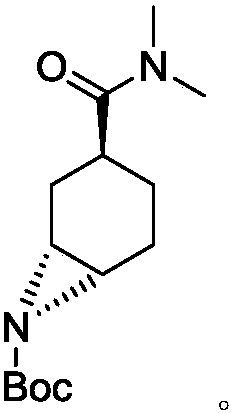
Preparation method for optically active diamino derivative
A kind of amino, amino protecting group technology, applied in the field of diamino derivatives, to achieve significant technical effect, high optical purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]
[0068] Put 2.2g of compound D, 22ml of toluene, and 1.3g of benzylamine into a four-necked flask, raise the temperature to 80-90°C, and keep it warm for 4-6 hours. Concentrate to dryness to obtain 3.2 target product F1 with a yield of 90%.
Embodiment 2
[0070]
[0071] Put 5 g of compound F1, 50 ml of ethanol, 5 ml of water, and 23 ml of 30% sodium hydroxide into a four-necked bottle. Heating up to 40-45°C, add 5g BOC dropwise 2 O acid anhydride, after dropping, react for 4-6 hours. After the reaction, concentrate to dry ethanol, add 50ml of EA, 30ml of water, separate layers, wash the organic layer with 10ml of saturated brine, dry 5g of sodium bicarbonate, filter with suction, and concentrate to dryness to obtain 6.1g of the target product F with a yield of 91%. , with a purity of 98%.
Embodiment 3
[0073]
[0074] Put 2.5g of compound F, 2g of phthalimide, 4g of triphenylphosphine, and 50ml of tetrahydrofuran into a four-necked bottle, and lower the temperature to 0-5°C under the protection of nitrogen, and add 2.4g of diethyl azodicarboxylate dropwise. After dripping and keeping warm for 4-6 hours, the reaction is complete. Add 20ml of water, 30ml of EA to extract the organic layer, wash the organic layer with 10ml of saturated brine, dry 3g of sodium bicarbonate, filter with suction, and concentrate the filtrate to dryness to obtain 4g of compound H with a yield of 82% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com