Application of active component of sanguisorba officinalis to preparation of antitumor medicines
An anti-tumor, multi-purpose technology, applied in the field of medicine, can solve unseen problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
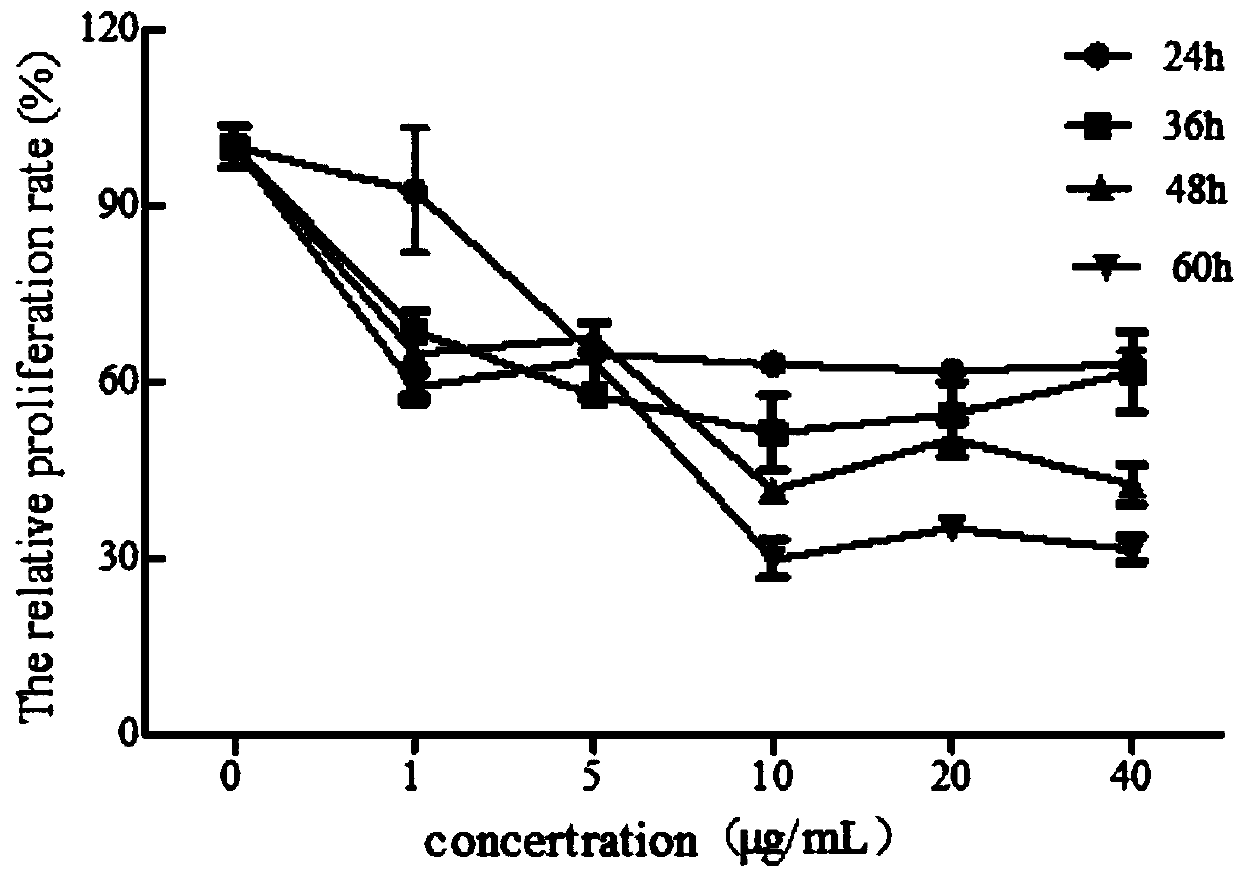
[0052] Example 1 The effect of 3,3',4'-trimethoxy inverse gallic acid on inhibiting the proliferation of human umbilical vein endothelial cells HUVEC cells
[0053] With 5% FBS (fetal bovine serum, Fetal bovine serum, Gibco, specification: 500mL / bottle, batch number: 42Q9462K), 1% penicillin-streptomycin mixture (Beyotime, specification: 100mL / bottle, batch number: C0222), 1 % Endothelial cell growth factor ECGS ECM complete medium (Sciencell, specification: 500mL / bottle, batch number: 1001), at 37 ° C, 5% CO 2 HUVEC cells were cultured in an incubator, and the medium was changed every other day. When the cell confluence was about 90%, the 1:2 subculture was carried out.
[0054] Take HUVEC cells in good growth state and digest them with trypsin, take 10 μL of cell suspension and count on the counting plate, adjust the cell density to 3×10 4 cells / mL, 3000 cells / well were inoculated in a 96-well plate, 100 μL per well. After the cells adhered to the wall, the medium was remo...
Embodiment 2
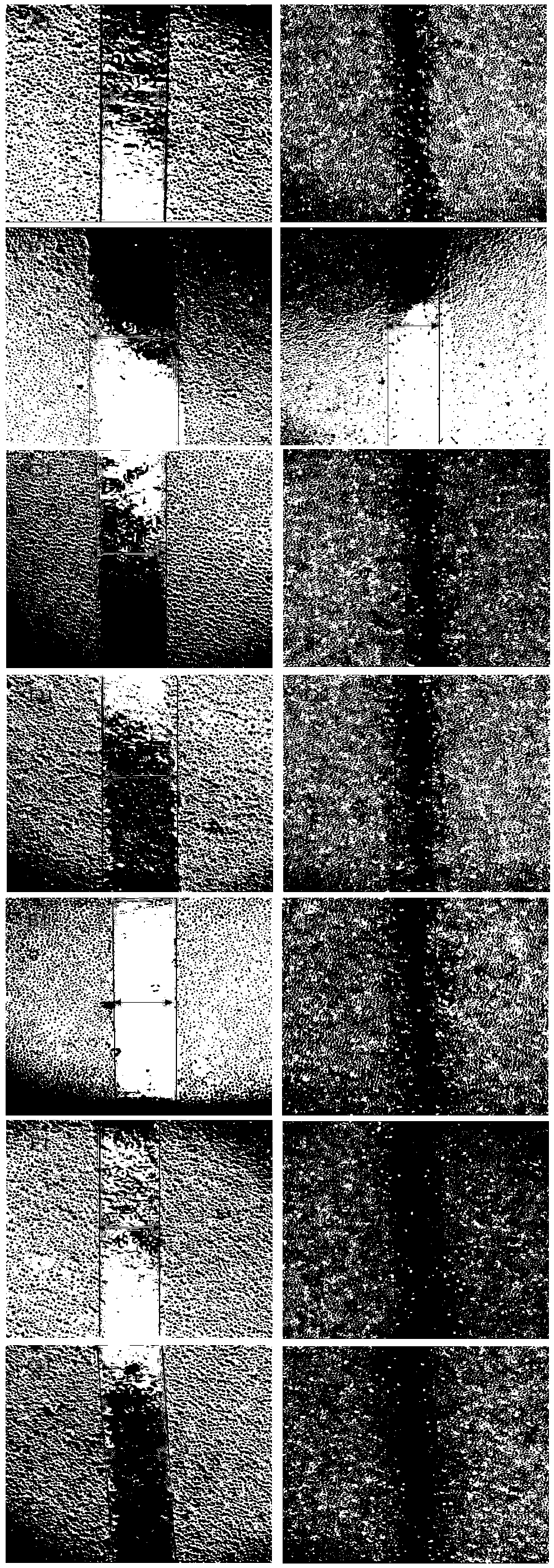
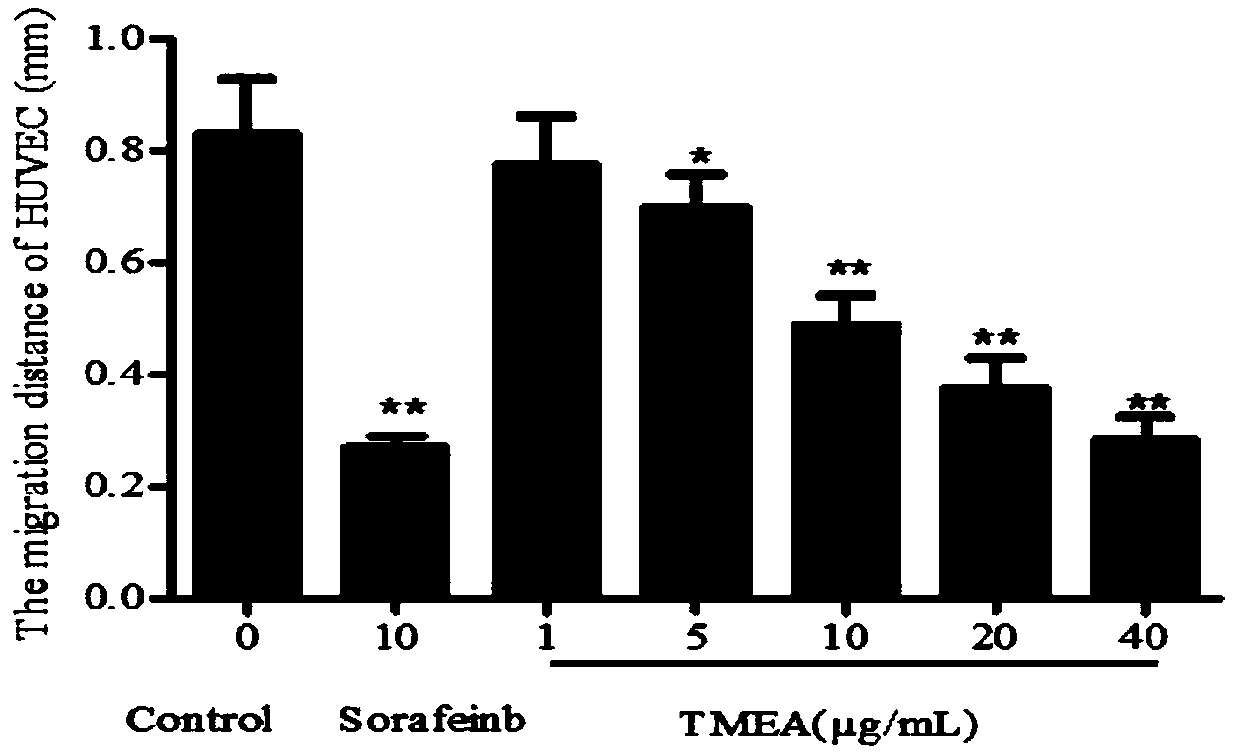
[0057] Example 2 The effect of 3,3',4'-trimethoxy inverse gallic acid on inhibiting the migration of human umbilical vein endothelial cells HUVEC cells
[0058] The effect of TMEA on the migration ability of HUVEC cells was investigated by scratch test. The Marker pen, ruler, 200 μL sterile pipette tip and other experimental equipment used in the experiment were placed in the ultra-clean bench for 30 minutes of ultraviolet irradiation in advance. The specific experimental steps are as follows, repeated three times:
[0059] Before cell inoculation, use a Maker pen to evenly draw 3 horizontal lines on the back of the 6-well plate, and wait for the ink to dry. After digesting HUVEC cells in good growth state with trypsin digestion solution, count on the counting plate and adjust the cell density to 1×10 6 Cells / mL were inoculated in a 6-well plate, 1 mL per well, the cells were evenly spread on the bottom of the 6-well plate, and cultured in an incubator for 24 hours.
[0060]...
Embodiment 3
[0067] Example 3 The effect of 3,3',4'-trimethoxy retrogallic acid on inhibiting tubule formation of human umbilical vein endothelial cells HUVEC cells in vitro
[0068] The Martrigel matrigel was liquefied overnight in a refrigerator at 4°C in advance, and all pipette tips used in the experiment had to be liquefied in a refrigerator at 4°C overnight to avoid uneven glue surfaces. During the experiment, Matrigel was always kept on ice to avoid its solidification affecting its use.
[0069] Spread 50 μL of liquefied Martrigel matrigel in each well of a 96-well plate, shake slightly on ice to evenly distribute it on the bottom of the 96-well plate, and then transfer it to a 37°C incubator for 30 minutes to solidify. After Matrigel was fully solidified, 2×10 5 cells / mL of the drug-containing cell suspension, after incubation in the incubator, observe the tubule formation under a 40× microscope. A blank group, a sorafenib group (10 μmol / mL) and five different concentrations (40,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com