Butylphthalide hard capsule, and preparation method thereof
A technology of butylphthalide and hard capsules, which is applied in the directions of capsule delivery, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problem of disintegration and the lack of obvious improvement in the level of related substances, and the air permeability of the hard capsules. High, gelatin cross-linking has not been solved and other problems, to achieve the effect of suitable industrialization promotion, avoid degradation and deterioration, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
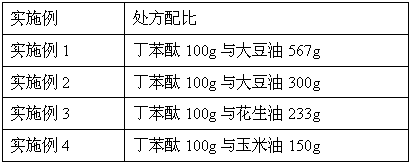
[0045] prescription:
[0046]
[0047] Preparation method:
[0048] (1) Weigh butylphthalide and oily base respectively according to the prescription and ratio in the above table, mix and stir evenly, and make mixed oil for later use;
[0049] (2) Take a commercially available hard capsule shell made of hydroxypropyl starch (substitution degree 1.8%), fill the mixed oil described in step (1) into the capsule body of the hard capsule shell through a liquid filling machine, and fit Capsule caps, adjust the filling capacity by adjusting equipment, and make 1000 hard capsules with oily contents;
[0050] (3) Dissolve Eudragit L100 in 85% ethanol aqueous solution to make a sealing material solution;
[0051] (4) Use the sealing material solution to spray circularly on the joint of the capsule body and capsule cap of the above-mentioned hard capsule with oily content, and dry to form a sealing film.
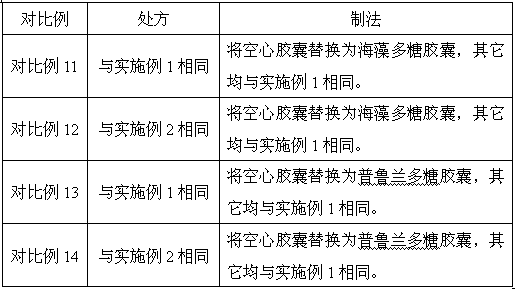
Embodiment 5~8
[0053] Prescription: same as Example 2.
[0054] Preparation method: except that the following factors change, other preparation methods are the same as in Example 2.
[0055]
Embodiment 9
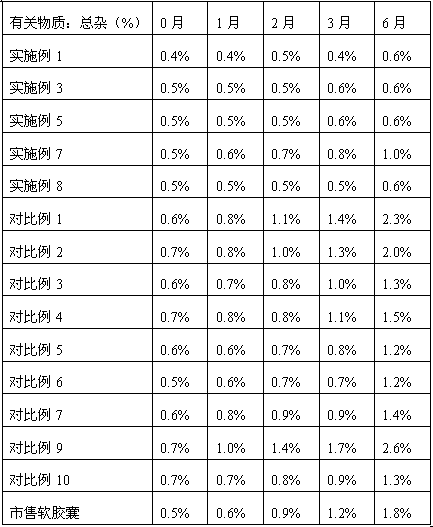
[0083] Carry out accelerated stability test simultaneously to the butylphthalide hard capsule that embodiment 1~8 makes and commercially available butylphthalide soft capsule, the result is as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com