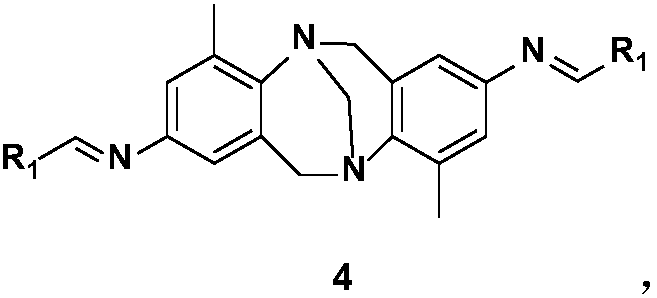
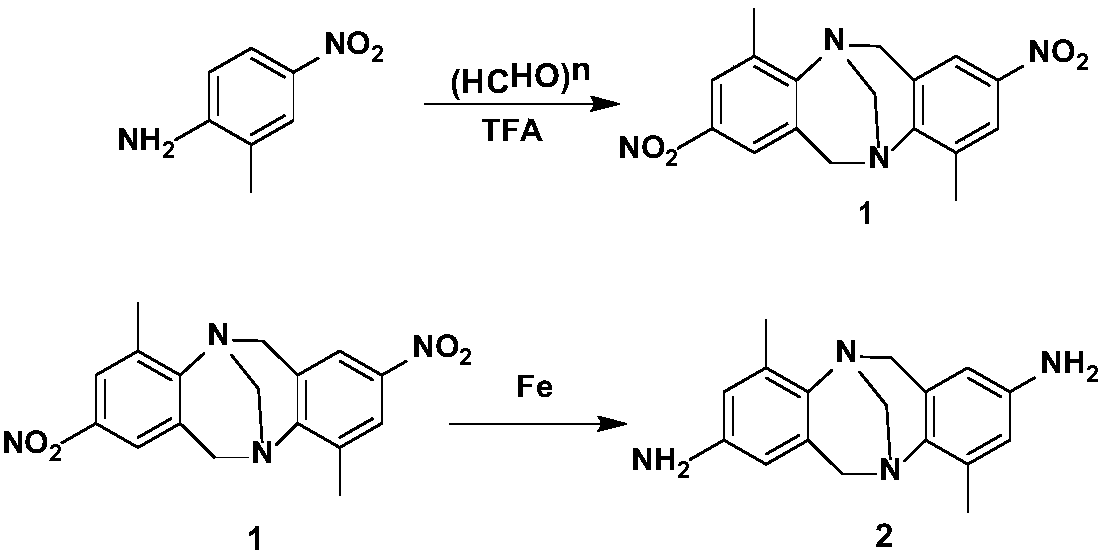
Bi-heterocycle aldolase-2,8-diamino-Troger's Base Schiff base catalyst and preparation method
A -base, two-heterocycle technology, applied in the field of biheterocyclic aldehyde acetal -2,8-diamino-Base Schiff base catalyst and preparation, to achieve the effects of enhancing alkalinity, improving yield and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: preparation compound 4a
[0036] Intermediate 2 (1.0mmol), 2-thiophenecarbaldehyde (1.2mmol), triethylamine (1mol%) and absolute ethanol (5mL) were successively added to a 50mL dry round-bottomed flask, refluxed at 80°C for 12h, and the reaction was completed ( TLC track), filter, evaporate excess solvent under reduced pressure, crude product is passed through column chromatography (V 石油醚 :V 乙酸乙酯 =1:9 gradient elution) separation to obtain compound 4a with a yield of 93%.
[0037] The structural formula of compound 4a is:
[0038] The molecular formula is: C 27 h 25 N 4 S 2
[0039] Chinese name: (1E,1'E)-N,N'–(4,10-dimethyl-6H,12H-5,11-methylenedibenzo[b,f][1,5] Diazaoctine-2,8-diyl)bis(1-(thien-2-yl)methimine)
[0040] English name: (1E,1'E)-N,N'-(4,10-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diyl )bis(1-(thiophen-2-yl)methanimine)
[0041] Appearance: yellow solid
[0042] Melting point: 212.5-214.2°C
[0043] Proton NMR...
Embodiment 2
[0046] Embodiment 2: preparation compound 4b
[0047] Add intermediate 2 (1.0mmol), 3-methylthiophene-2-carbaldehyde (1.2mmol), triethylamine (1mol%) and absolute ethanol (5mL) successively into a 50mL dry round bottom flask, and reflux at 80°C 12h, the reaction finishes (TLC follow-up), filters, evaporates excess solvent under reduced pressure, and crude product is passed through column chromatography (V 石油醚 :V 乙酸乙酯 =1:9 gradient elution) separation to obtain compound 4b with a yield of 91%.
[0048] The structural formula of compound 4b is:
[0049] The molecular formula is: C 29 h 29 N4 S 2
[0050] Chinese name: (1E,1'E)-N,N'-(4,10-dimethyl-6H,12H-5,11-methylenedibenzo[b,f][1,5 ]diazaoctine-2,8-diyl)bis(1-(3-methyl-thiophen-2-yl)methimine)
[0051] English name: (1E,1'E)-N,N'-(4,10-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diyl )bis(1-(3-methyl-thiophen-2-yl)methanimine)
[0052] Appearance: yellow solid
[0053] Melting point: 231.6-233.8°C ...
Embodiment 3
[0057] Embodiment 3: preparation compound 4c
[0058] Add intermediate 2 (1.0mmol), 5-methylthiophene-2-carbaldehyde (1.3mmol), triethylamine (1mol%) and absolute ethanol (5mL) successively into a 50mL dry round bottom flask, and reflux at 80°C 12h, the reaction finishes (TLC follow-up), filters, evaporates excess solvent under reduced pressure, and crude product is passed through column chromatography (V 石油醚 :V 乙酸乙酯 =1:9 gradient elution) separation to obtain compound 4c with a yield of 88%.
[0059] The structural formula of compound 4c is:
[0060] The molecular formula is: C 29 h 29 N 4 S 2
[0061] Chinese name: (1E,1'E)-N,N'-(4,10-dimethyl-6H,12H-5,11-methylenedibenzo[b,f][1,5 ]diazaoctine-2,8-diyl)bis(1-(5-methyl-thiophen-2-yl)methimine)
[0062] English name: (1E,1'E)-N,N'-(4,10-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diyl )bis(1-(5-methyl-thiophen-2-yl)methanimine)
[0063] Appearance: yellow solid
[0064] Melting point: 178.3-179.9°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com