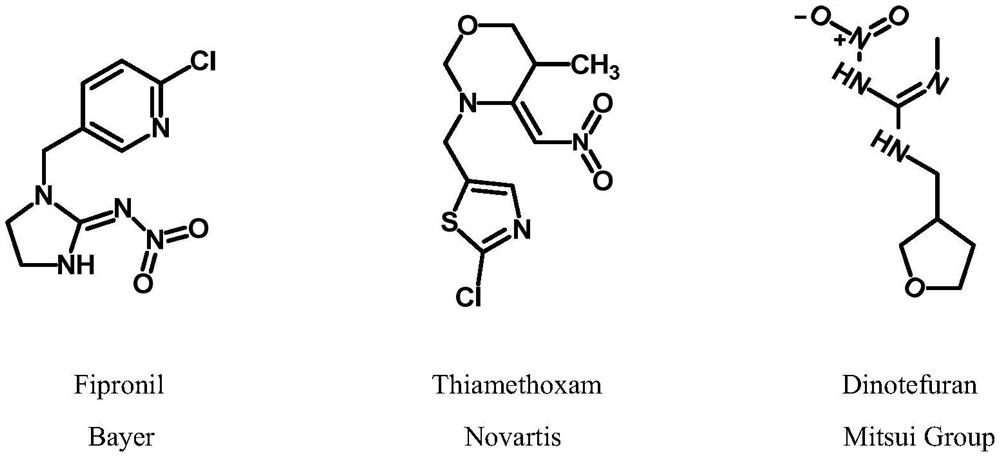
1,2,4-triazole compounds and their salts and applications
A compound and triazole technology, applied in the field of 1,2,4-triazole compounds and their salts and applications, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
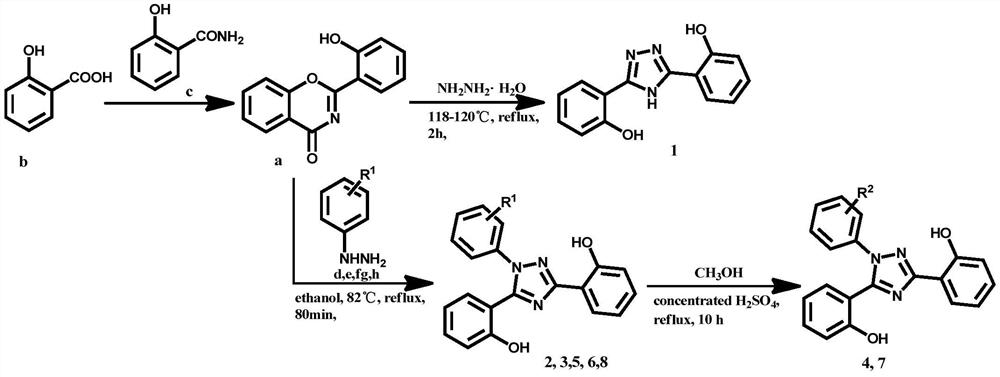
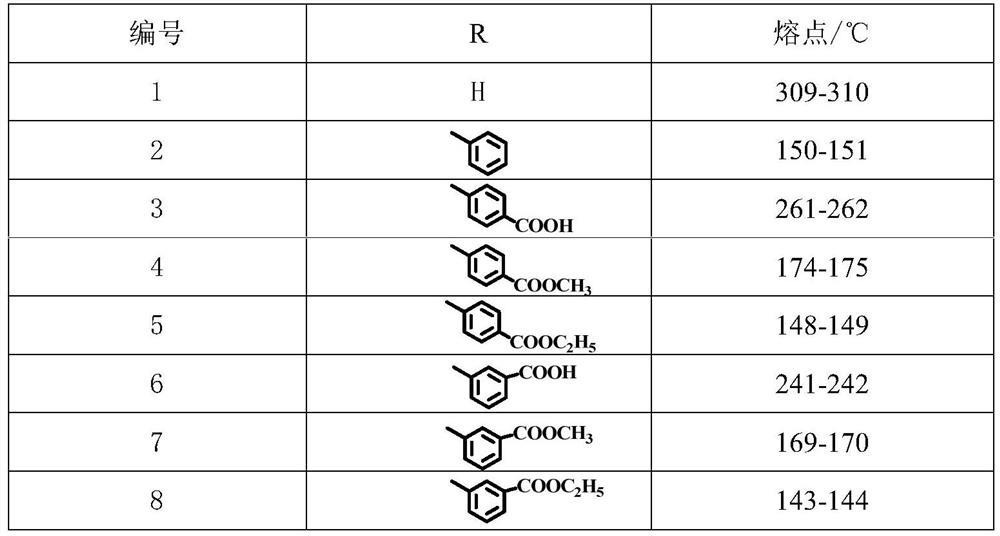
[0027] Preparation of 3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazole (1)
[0028] Add 0.5g of key intermediate a and 20mL of hydrazine hydrate to a 100mL three-necked flask in turn, and heat to reflux for 2 hours. After the reaction, add an appropriate amount of water to the reaction solution, and a white solid precipitates out. Filter and recrystallize with ethanol to obtain a powder Solid, melting point 309-310°C.
Embodiment 2
[0030] Preparation of 3,5-bis(2-hydroxyphenyl)-1-phenyl-1,2,4-triazole (2)
[0031] In a 150mL three-necked flask, add 20mL of absolute ethanol, 1mL of phenylhydrazine (d), 0.5g of key intermediate a, and 0.5mL of acetic acid as a catalyst. Heated to reflux for 2 hours. After the reaction, the solvent was removed under reduced pressure, and the residue was purified by column chromatography to obtain a white solid with a melting point of 150-151°C.
Embodiment 3
[0033] Preparation of 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoic acid (3)
[0034] Dissolve 1.75g of 4-hydrazinobenzoic acid (e) and 1.16g of NEt3 completely in 30mL of hot ethanol solution, add 2.50g of key intermediate a, and heat to reflux for 2-3h. After the reaction, concentrate under reduced pressure to half the volume, add an appropriate amount of water, adjust the pH to 5 with hydrochloric acid, a white floc is formed, and filter and purify to obtain a light yellow solid with a melting point of 261-262°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com