Method for preparing self-curable phthalonitrile resin with triphenol A structures
A technology of phthalonitrile and nitrophthalonitrile, applied in the field of material science, can solve problems such as resin defects, and achieve the effects of broad application prospects, excellent melt processing fluidity, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
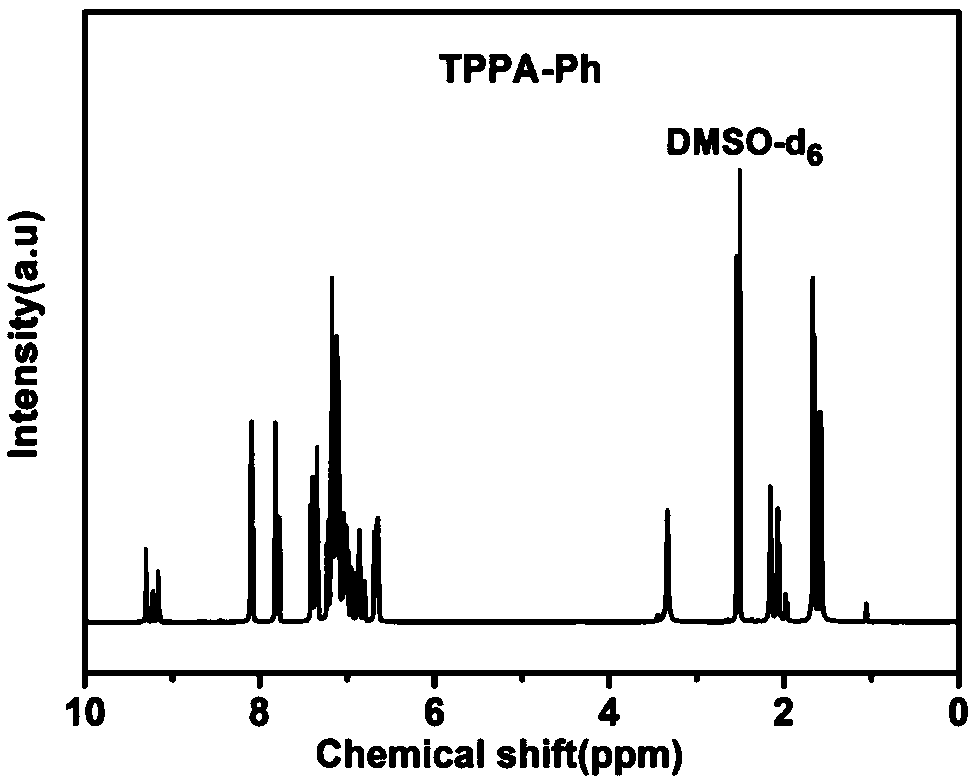
[0022] Monomer synthesis: Add 10g of trisphenol A, 4.88g of catalyst anhydrous potassium carbonate and 4.28g of 4-nitrophthalonitrile into the reactor, then add 100mL of dried dimethyl sulfoxide, and then nitrogen Protected and reacted at 25°C for 24 hours. After the reaction, pour the product into 1L of deionized water, then adjust the pH value of the system to neutral with 1mol / L dilute hydrochloric acid, then wash with deionized water until the filtrate is neutral, then vacuum dry at 70°C for 24 hours , to obtain the phthalonitrile monomer (TPPA-Ph) containing triphenol A structure, and set aside.
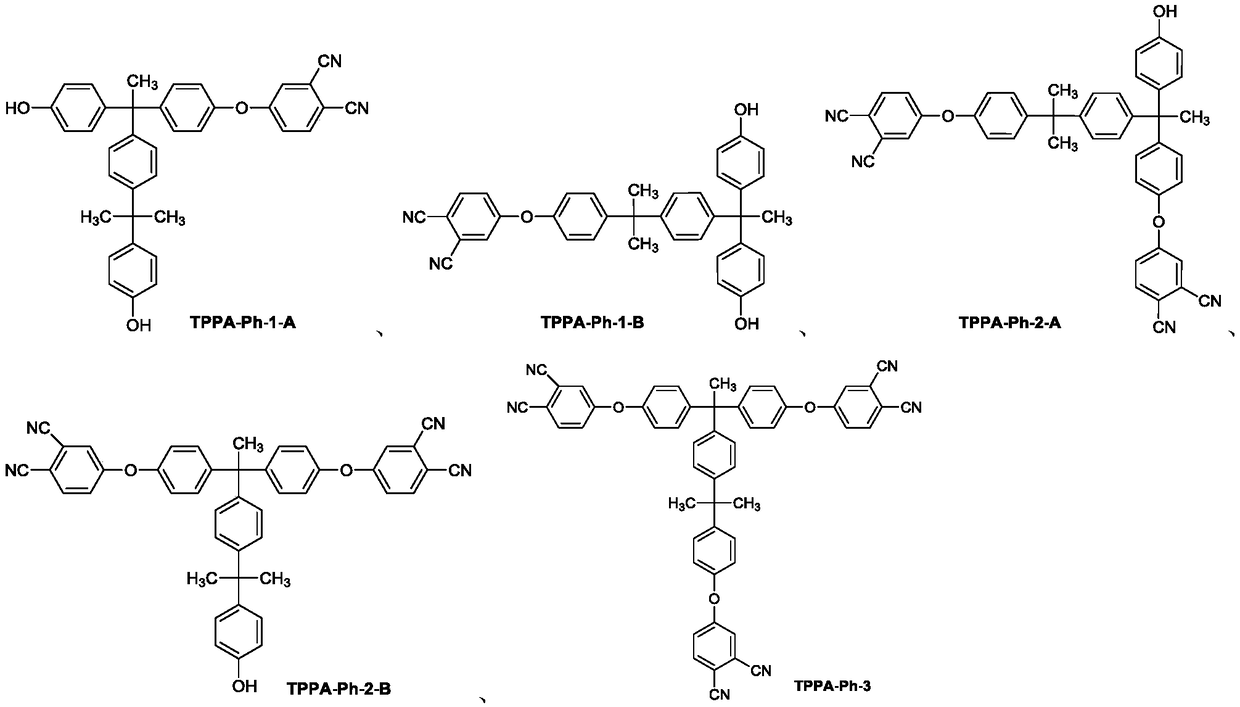
[0023] Curing: take the above-mentioned 1g dried triphenol A type self-curing phthalonitrile precursor (component content TPPA-Ph-1 is 44.57%, TPPA-Ph-2 is 42.85% and TPPA-Ph-3 is 12.58%), after being fully ground, put it into a reactor with a temperature of 100°C, heat and stir until it is completely melted, then pour the liquid mixture into a mold, and heat it in a muffle fur...
Embodiment 2
[0027] Monomer synthesis: Add 10g of trisphenol A, 4.88g of catalyst anhydrous potassium carbonate and 8.36g of 4-nitrophthalonitrile into the reactor, then add 100mL of dried dimethyl sulfoxide, and then nitrogen protection, reacted at 25°C for 36 hours. After the reaction, pour the product into 1L of deionized water, then adjust the pH value of the system to neutral with 1mol / L dilute hydrochloric acid, then wash with deionized water until the filtrate is neutral, then vacuum dry at 70°C for 24 hours , to obtain the phthalonitrile monomer (TPPA-Ph) containing triphenol A structure, and set aside.
[0028]Curing: take the above-mentioned 1g dried triphenol A type self-curing phthalonitrile precursor (component content TPPA-Ph-1 is 11.64%, TPPA-Ph-2 is 42.37% and TPPA-Ph-3 is 45.99%), after being fully ground, put it into a reactor with a temperature of 100°C, heat and stir until completely melted, then pour the liquid mixture into a mold, and heat it in a muffle furnace at 2...
Embodiment 3
[0031] Monomer synthesis: Add 10g of trisphenol A, 8.14g of catalyst anhydrous sodium carbonate and 13.05g of 4-nitrophthalonitrile into the reactor, then add 100mL of dried dimethyl sulfoxide, and then nitrogen protection, reacted at 80°C for 48 hours. After the reaction is over, pour the product into 1L deionized water, adjust the pH value of the system to neutral with 1mol / L dilute hydrochloric acid after cooling down to room temperature, and then wash with deionized water until the filtrate is neutral, then vacuum at 70°C Dry for 24 hours to obtain a phthalonitrile monomer (TPPA-Ph) containing a triphenol A structure, which is ready for use.
[0032] Curing: take the above-mentioned 1g dried trisphenol A type self-curing phthalonitrile precursor (component content TPPA-Ph-1 is 0%, TPPA-Ph-2 is 9.79% and TPPA-Ph-3 is 90.21%), after being fully ground, put it into a reactor with a temperature of 100°C, heat and stir until completely melted, then pour the liquid mixture into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com