Hydrogen bond organic framework material based on condensed ring ligand construction as well as preparation method and application thereof
An organic framework and ligand technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of inability to maintain permanent pores and poor stability, and achieve excellent singlet oxygen The effect of production ability, mild synthesis conditions, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
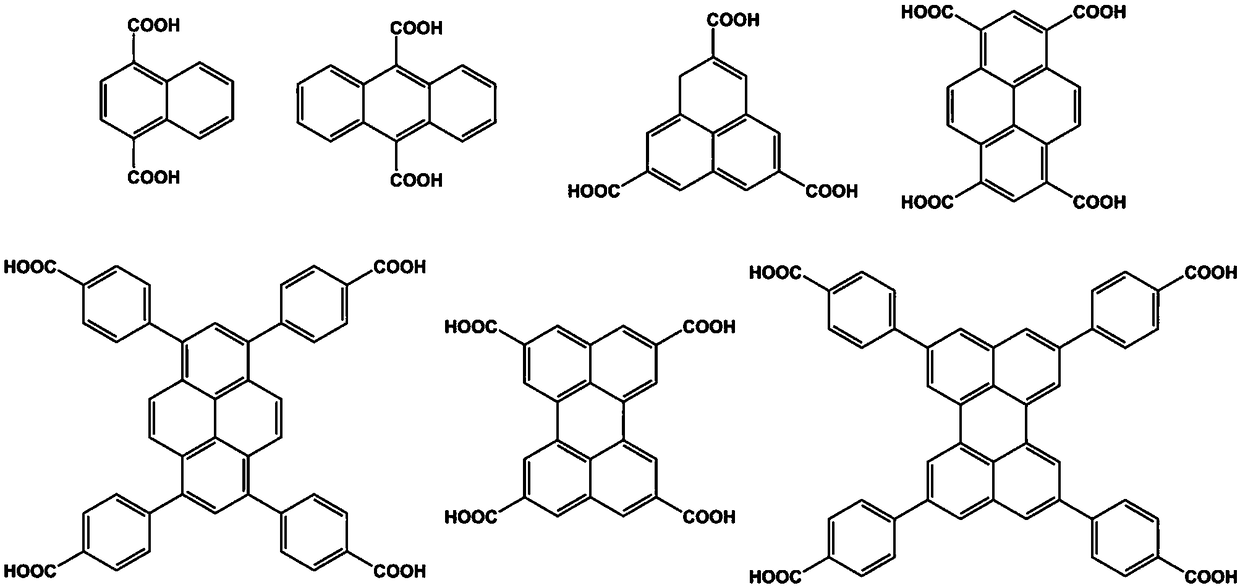
[0060] Example 1 Hydrogen-bonded organic framework material based on 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene
[0061] The preparation process of hydrogen-bonded organic framework materials based on 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene:
[0062] Dissolve 10 mg of 1,3,6,8-tetrakis(p-carboxyphenyl)perylene in 1.5 ml of N,N-dimethylformamide. After the ligand is completely dissolved, add 6 ml of methanol solution to the solution , stirred for 1 minute, after which the samples were allowed to stand at room temperature for 12 hours. Afterwards, centrifuge at a speed of 12000 rpm for 3 minutes, and wash with acetone for 3 consecutive times to obtain a hydrogen-bonded organic framework material based on 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene. The material has a high specific surface area: 2122 m2 / g calculated according to the Brunauuer-Emmett-Teller formula, with a pore size of 1.7 nm.
[0063] This material can still be obtained after expanding the proportions of the above mate...
Embodiment 2
[0064] Embodiment 2 Hydrogen-bonded organic framework materials based on naphthalene, anthracene, perylene, perylene
[0065] The preparation process of the hydrogen-bonded organic framework material based on naphthalene, anthracene, perylene, and perylene is the same as in the examples, only the raw materials need to be replaced by figure 2 Specific compounds shown.
Embodiment 3
[0066] Example 3 Hydrogen-bonded organic framework material based on 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene at the micro-nano level
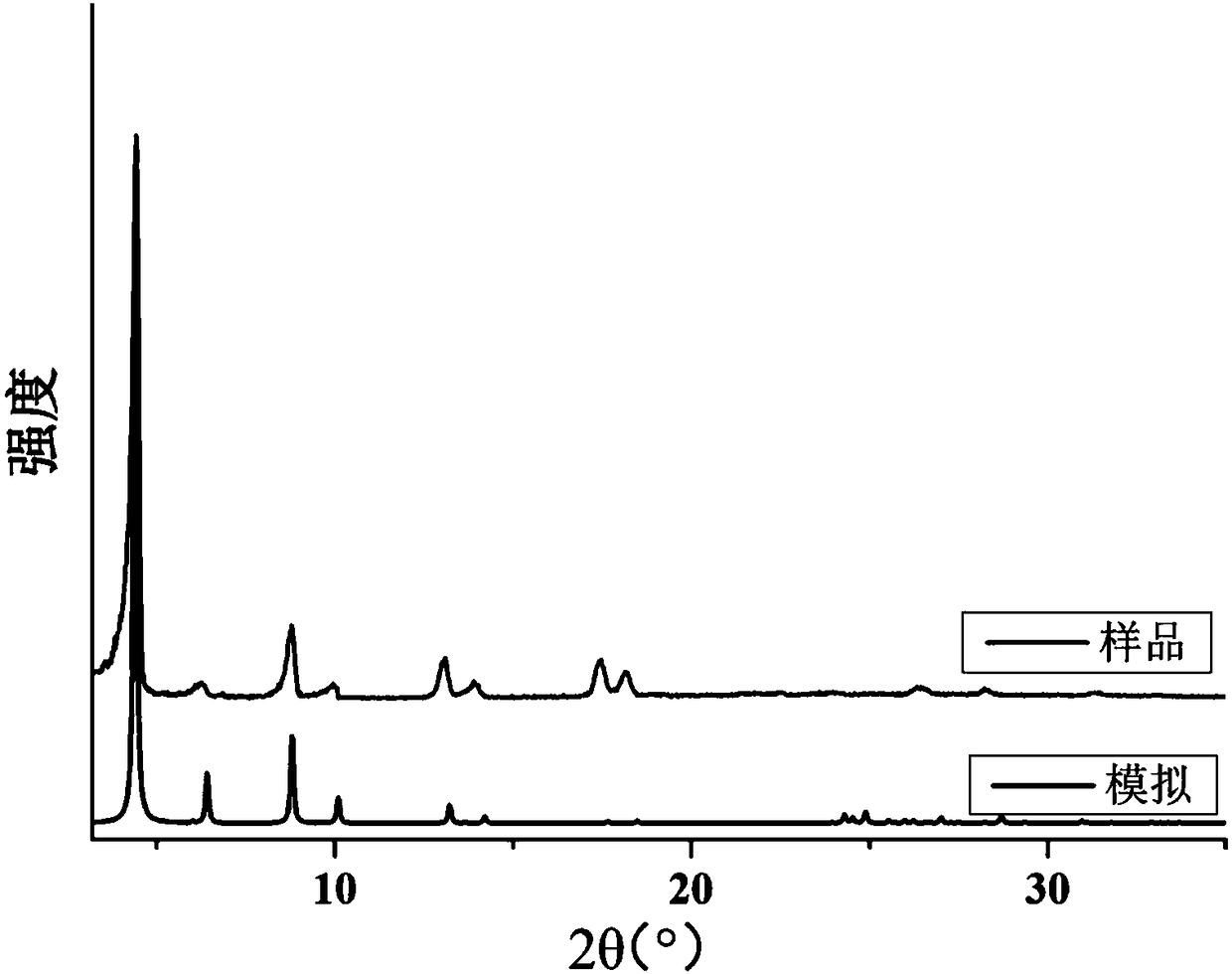
[0067] The preparation process of nanoscale hydrogen-bonded organic framework materials based on 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene: 10 mg of 1,3,6,8-tetrakis(p-carboxyphenyl)pyrene was dissolved in After the ligand was completely dissolved in 1.5 ml of N,N-dimethylformamide, 10 ml of deionized water was added to the solution at a stirring speed of 1000 rpm, and kept stirring for 5 minutes. Then add 9 milliliters of ethanol, and centrifuge at a speed of 12,000 rpm for 15 minutes, and wash with ethanol and acetone continuously for 4 times to obtain a pyrene-based hydrogen-bonded organic framework material with a particle size of 200 nanometers (see Figure 8 d).
[0068] By changing the reaction time and conditions of the synthesis, the hydrogen-bonded organic framework material from the micron scale to the nano scale can be obtained:
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com