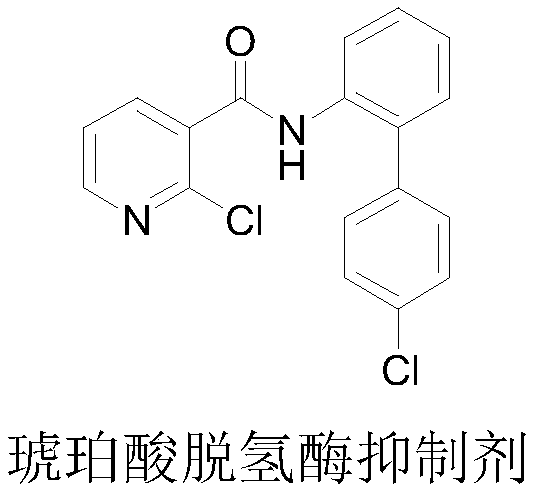
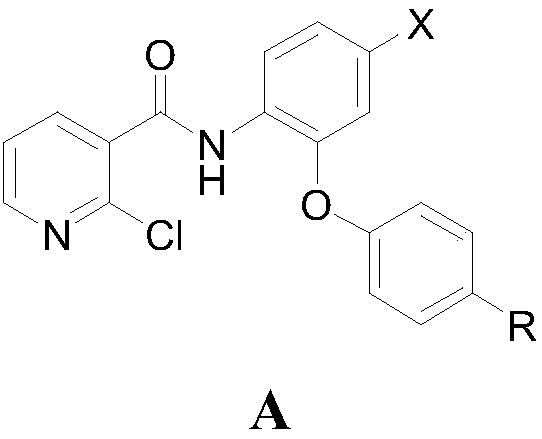
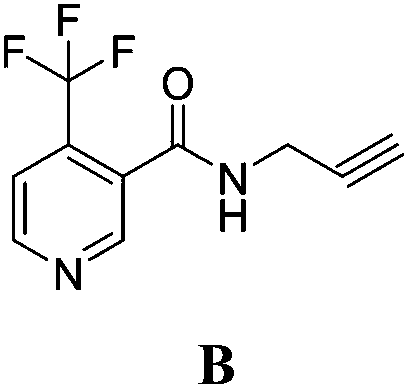
4-bromine-2-picolinamide compound containing diphenyl ether structure
A technology of pyridine amides and diphenyl ethers, applied in the field of new compounds, can solve the problems of reduced insecticidal and bacteriostatic effect, large chemical pollution and high toxicity and side effects, and achieves the effects of low toxicity, simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of a 4-bromo-2-pyridine amide compound containing a diphenyl ether structure, comprising the steps of:
[0033] (1) Dissolve 2-phenoxynitrobenzene (0.215g, 1.0mmol) in 30ml ethanol, add 80% hydrazine hydrate (0.068g, 1.0mmol) under stirring, heat up to reflux, react for 6.0 hours, cool, and filter , to obtain a brown solid, the intermediate 2-phenoxyaniline, with a yield of 90%.
[0034] (2) Dissolve 2-phenoxyaniline (0.185g, 1.0mmol) and 4-bromo-2-pyridinecarboxylic acid (0.202g, 1.0mmol) generated in step (1) in 25ml of dichloromethane, add three Ethylamine (0.202g, 2.0mmol), under the condition of the catalyst DMAP, then added EDCI (0.287g, 1.5mmol), HOBt (0.20g, 1.5mmol), reacted at 25°C for 6 hours, TLC detected that the reaction was complete, and the reaction solution Washed three times with water, washed once with saturated brine, dried the organic layer with anhydrous sodium sulfate, desolvated, the crude product was column chromatographed ...
Embodiment 2
[0037] A preparation method of a 4-bromo-2-pyridine amide compound containing a diphenyl ether structure, comprising the steps of:
[0038] (1) Dissolve 2-phenoxynitrobenzene (0.246g, 1.0mmol) in 30ml ethanol, add 80% hydrazine hydrate (0.073g, 1.0mmol) under stirring, heat up to reflux, react for 6.5 hours, cool, and filter , to obtain a brown solid, the intermediate 2-phenoxyaniline, with a yield of 92%.
[0039] (2) Under nitrogen protection, 25ml of 4-bromo-2-pyridinecarboxylic acid (0.214g, 1.0mmol) was dissolved in acetonitrile, and 2-phenoxyaniline (0.196g, 1.0mmol) and DIEA (0.389g , 3.0mmol), the temperature was lowered to below 10°C, the temperature was controlled at 0-10°C, and HBTU (0.493g, 1.30mmmol) was added rapidly, and the temperature was raised to room temperature for 8.0 hours. TLC detected that the reaction was complete and the solution was removed. The crude product was obtained by flash column chromatography as a dark green solid, namely 4-bromo-2-pyridi...
Embodiment 3
[0042] A preparation method of a 4-bromo-2-pyridine amide compound containing a diphenyl ether structure, comprising the steps of:
[0043] (1) Dissolve 2-phenoxynitrobenzene (0.214g, 1.0mmol) in 30ml ethanol, add 80% hydrazine hydrate (0.054g, 1.0mmol) under stirring, heat up to reflux, react for 7.0 hours, cool and filter , to obtain a brown solid, the intermediate 2-phenoxyaniline, with a yield of 91%.
[0044](2) Dissolve 32ml of 2-phenoxyaniline (0.174g, 1.0mmol) and 4-bromo-2-pyridinecarboxylic acid (0.232g, 1.0mmol) in dichloromethane, and quickly add DCC (0.206g, 1.0mmol) and DMAP (0.012g, 0.10mmol), stirred and reacted at room temperature for 8.0 hours. After the reaction was completed, washed twice with 30ml of saturated saline, once with 30ml of water, dried over sodium sulfate, and precipitated. The crude product was obtained by flash column chromatography. Green solid, namely 4-bromo-2-pyridine amide compound containing diphenyl ether structure, m.p.63-65°C, yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com