Method for removing chlorine in process of zinc electrolysis by ammonia method
An electrolytic zinc and process technology, which is applied to the field of chlorine removal in the ammonia electrolytic zinc process, can solve the problems of high chlorine removal cost of high-chlorine zinc-containing materials, blockage of electrolyte pipelines, large hardware investment, etc., and is conducive to popularization and application. , The operation process is stable, and the effect of each index reaching the standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
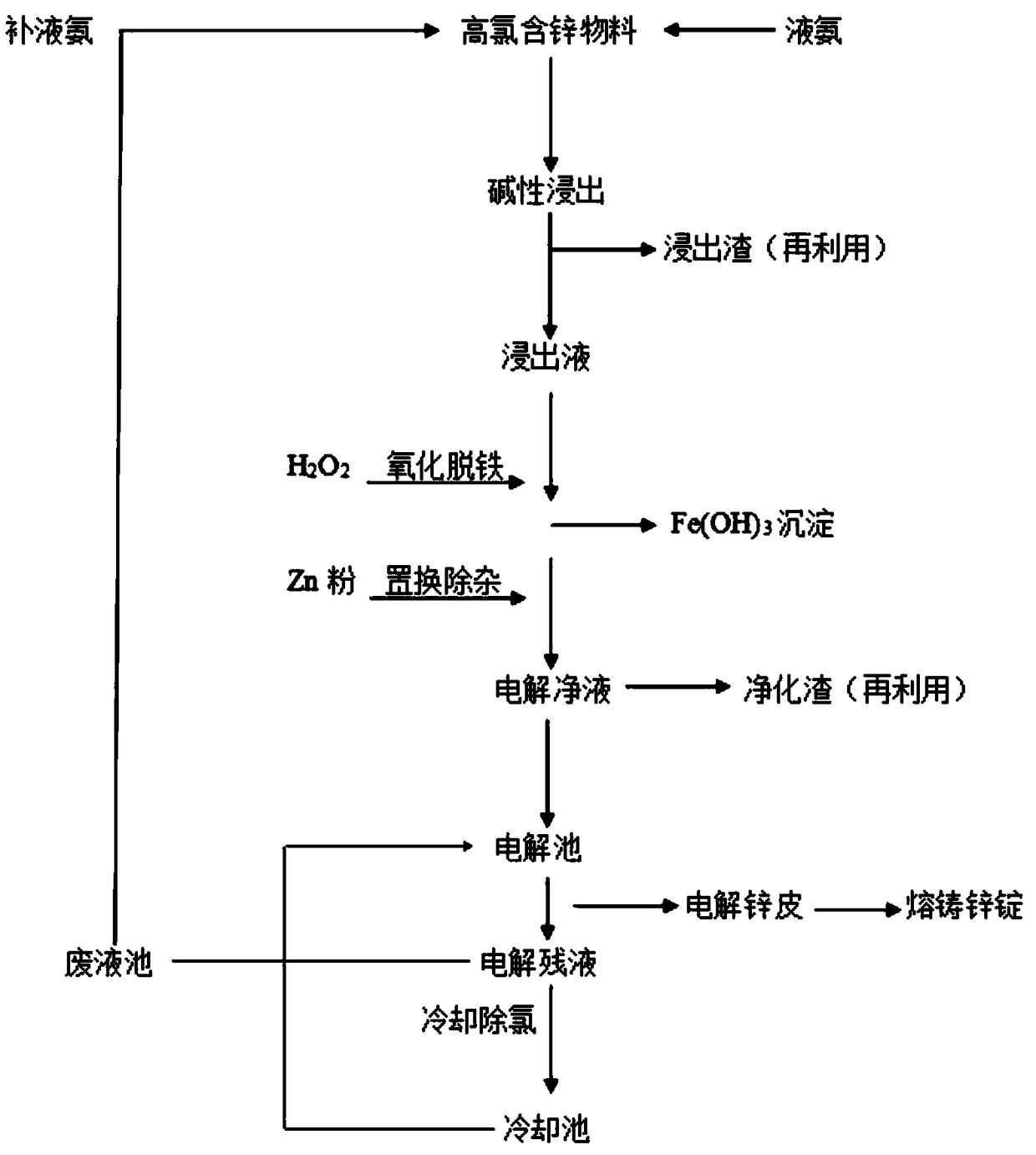
[0017] Such as figure 1 Shown, the method for removing chlorine in a kind of ammonia method electrolytic zinc process comprises following operation:
[0018] (1) Leaching: Alkaline leaching of high-chloride zinc-containing materials with liquid ammonia, the reaction temperature is 45°C-50°C, and the pH at the end of the reaction is controlled to be 8.0-8.5;
[0019] (2) Solid-liquid separation: The leached product is subjected to solid-liquid separation to obtain leaching liquid and leaching slag, which can be recycled and reused;
[0020] (3) Purification: Transport the leaching solution to a purification tank, add hydrogen peroxide for oxidative deferrification, then add zinc powder for replacement and removal of impurities, and control the temperature at the end of the reaction to 40°C to 45°C to obtain a purified electrolytic solution and purification slag, the purification slag can be recycled and reused;
[0021] (4) Electrolysis: The electrolytic net liquid is transpo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com