Soybean protein isolate low-residue comprehensive nutritional formula product and preparation method and application thereof
A soybean protein isolate and complete nutrition technology, applied in the field of special medical low-residue complete nutrition formula products, can solve problems such as difficult absorption, insufficient cellulose content, and increased intestinal air bubbles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
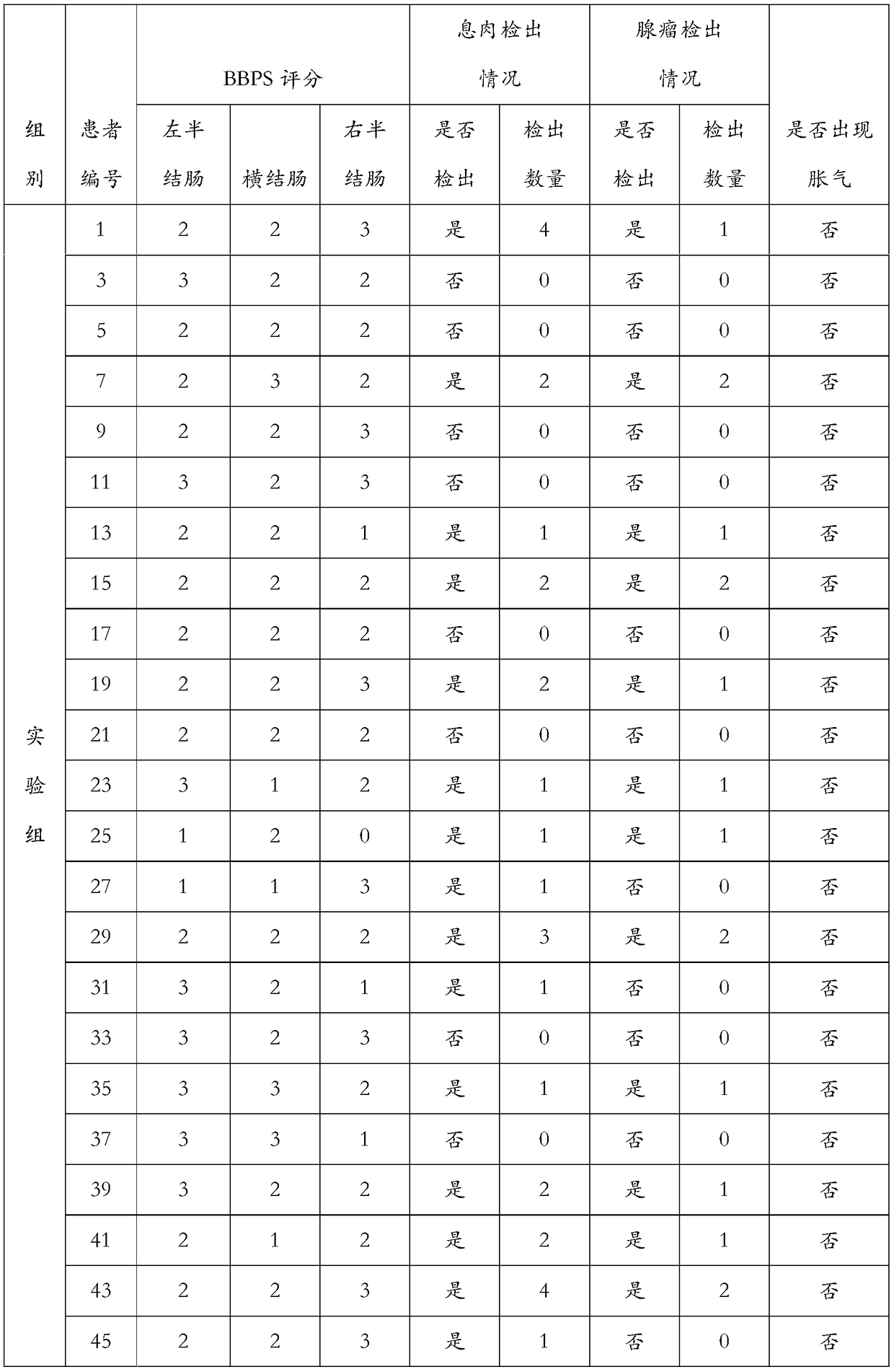
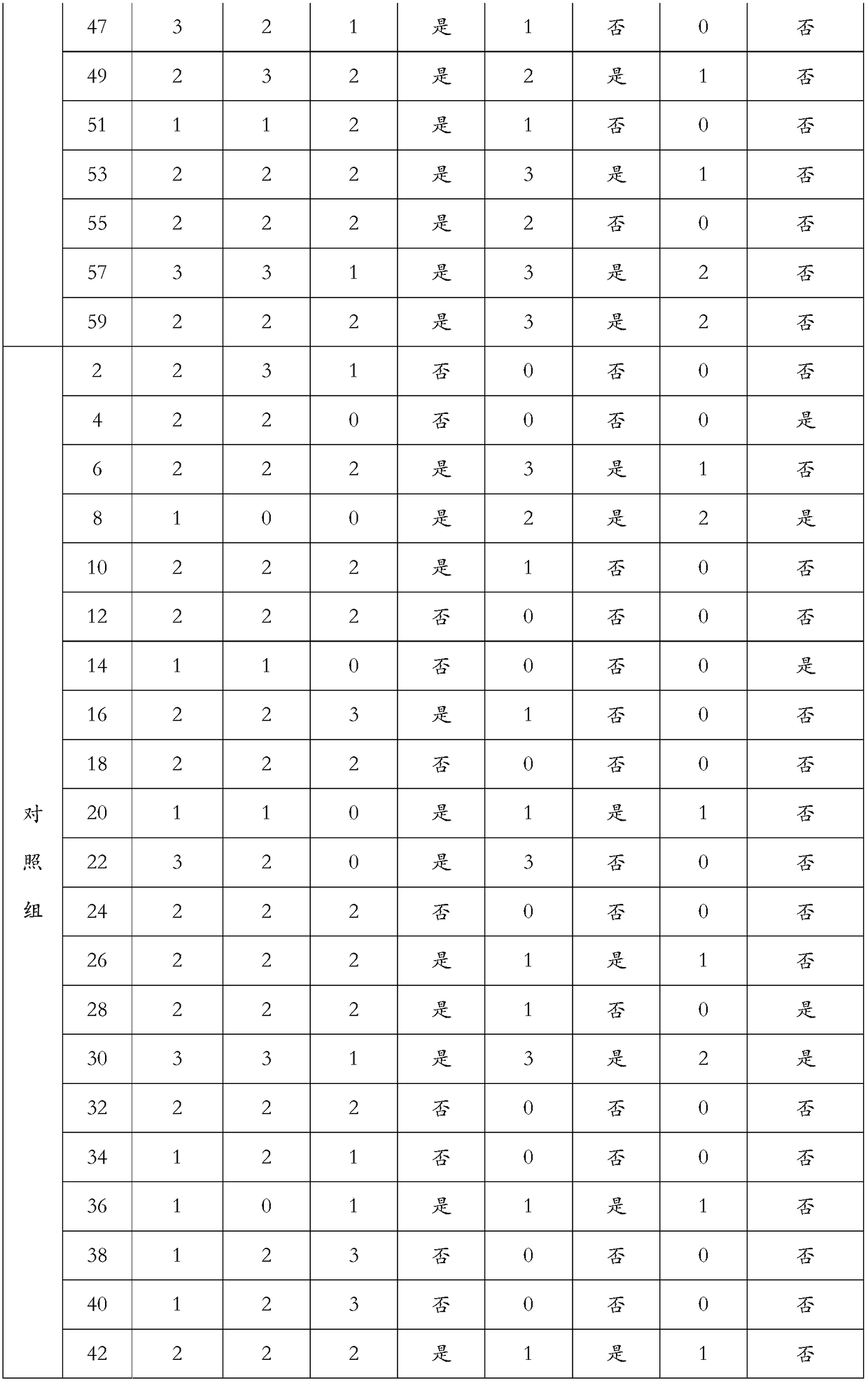
Embodiment 1
[0158] Example 1: Low residue full nutritional formula product 1
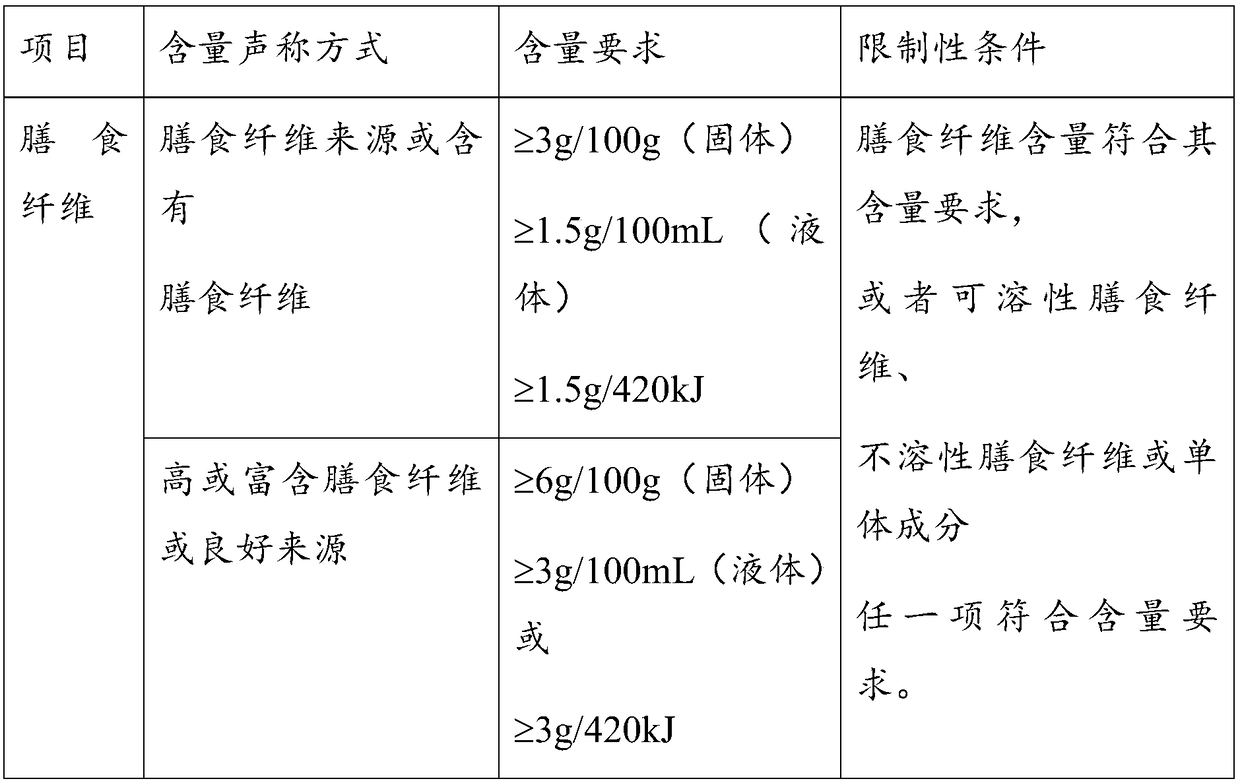
[0159] In every 1000g of the product in this example, as vitamins, it contains: 1.8g of sodium L-ascorbate, 0.23g of nicotinamide, 0.0023g of folic acid, 0.5g of dl-α-tocopheryl acetate, 0.1g of calcium D-pantothenate, and 0.17g of riboflavin g, pyridoxine hydrochloride 0.32g, thiamine hydrochloride 0.02g, retinyl acetate 0.06g, cholecalciferol 0.03g, phytonadione 0.0007g, cyanocobalamin 0.00007g, D-biotin 0.00016g.
[0160] In each 1000g product of this embodiment, as minerals, containing: ferrous sulfate 0.2g, zinc sulfate 0.2g, magnesium sulfate 15g, manganese sulfate 0.02g, potassium iodate 0.002g, copper sulfate 0.007g, sodium selenite 0.0006 g, 27g of potassium citrate, 7.2g of sodium citrate, 12g of potassium chloride, 4g of calcium hydrogen phosphate, 3.4g of sodium chloride and 2g of calcium carbonate. These minerals were mixed evenly with 20g of maltodextrin as embedding agent.
[0161] The amount o...
Embodiment 2
[0163] Embodiment 2: Low-residue full-nutrition formula product 2
[0164] In every 1000g of the product in this example, as vitamins, it contains: 1.2g of sodium L-ascorbate, 0.18g of nicotinamide, 0.0017g of folic acid, 0.4g of dl-α-tocopheryl acetate, 0.07g of D-calcium pantothenate, and 0.1g of riboflavin g, pyridoxine hydrochloride 0.2g, thiamine hydrochloride 0.012g, retinyl acetate 0.04g, cholecalciferol 0.03g, phytonadione 0.0005g, cyanocobalamin 0.00006g, D-biotin 0.00012g.
[0165] In every 1000g of the product in this embodiment, as minerals, it contains: ferrous sulfate 0.15g, zinc sulfate 0.15g, magnesium sulfate 13g, manganese sulfate 0.015g, potassium iodate 0.001g, copper sulfate 0.005g, sodium selenite 0.0004 g, 25g of potassium citrate, 6.5g of sodium citrate, 11g of potassium chloride, 3.6g of calcium hydrogen phosphate, 2.8g of sodium chloride and 1.8g of calcium carbonate. These minerals were mixed evenly with 20g of maltodextrin as embedding agent.
[...
Embodiment 3
[0168] Example 3: Low-residue full-nutrition formula product 3
[0169] Every 1000g of the product in this example contains, as vitamins: 1g of sodium L-ascorbate, 0.1g of nicotinamide, 0.001g of folic acid, 0.25g of dl-α-tocopheryl acetate, 0.06g of calcium D-pantothenate, and 0.1g of riboflavin , pyridoxine hydrochloride 0.2g, thiamine hydrochloride 0.012g, retinyl acetate 0.04g, cholecalciferol 0.02g, phytonadione 0.0005g, cyanocobalamin 0.00006g, D-biotin 0.0001g.
[0170] In every 1000g of the product in this embodiment, as minerals, it contains: ferrous sulfate 0.1g, zinc sulfate 0.2g, magnesium sulfate 10g, manganese sulfate 0.015g, potassium iodate 0.002g, copper sulfate 0.004g, sodium selenite 0.0005 g, 18g of potassium citrate, 5g of sodium citrate, 9g of potassium chloride, 2.8g of calcium hydrogen phosphate, 2.4g of sodium chloride and 1g of calcium carbonate, these minerals were mixed evenly with 20g of maltodextrin as embedding agent.
[0171] The amount of ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com