Dextrorotatory oxiracetam oral dispersing membrane and preparation method thereof
A technology of dextrooxypyridine and dispersing film, which is applied in the field of dextrooxypyridine pharmaceutical composition, can solve the problems of low drug loading, restrictions on the development and application of orodispersible films, disintegration time and resistance Tension strength is difficult to control and other problems, to achieve the effect of simple preparation method, improve bioavailability, and avoid elimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
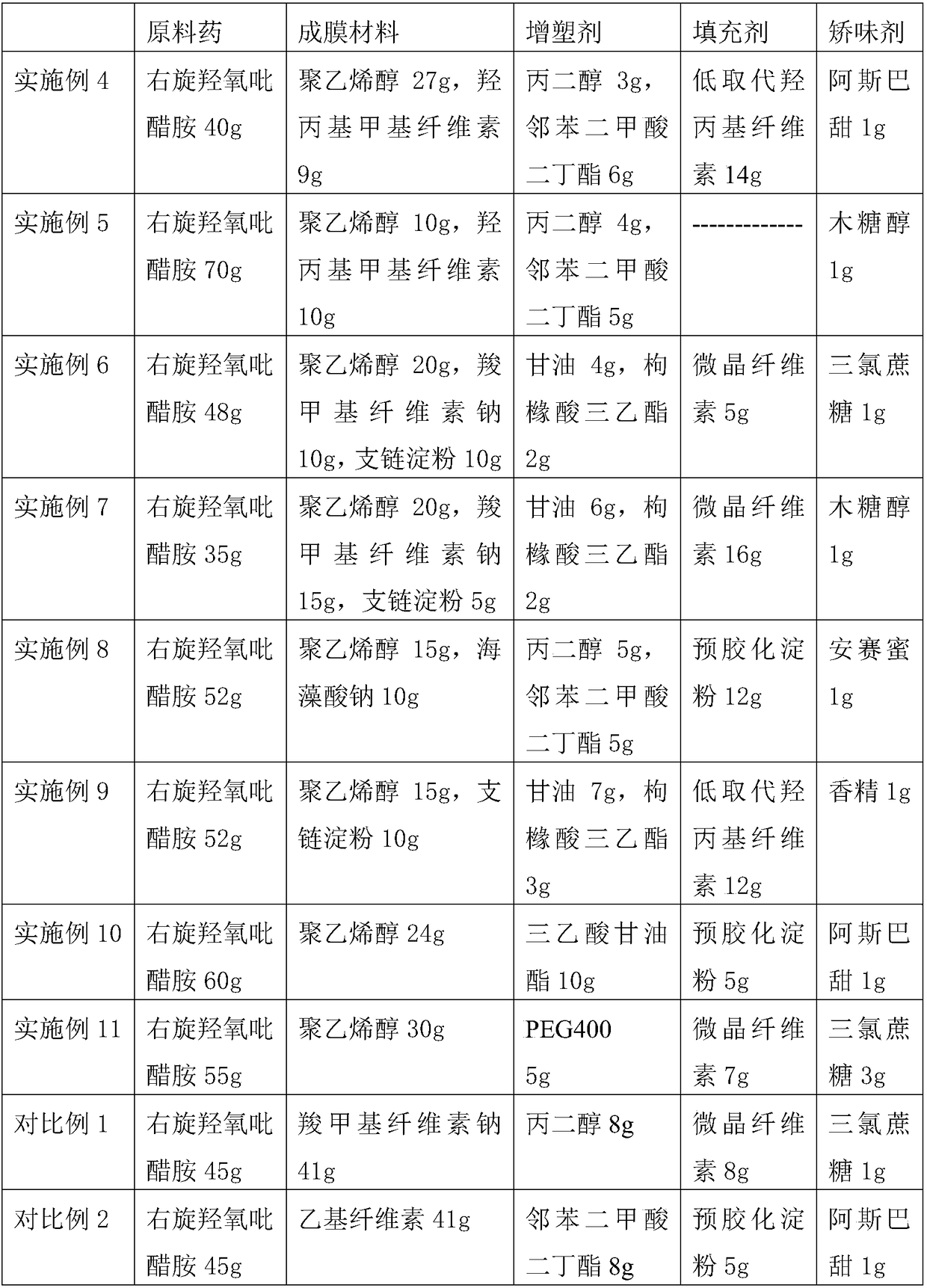
[0031] Prescription: 65g of dextrooxypyridine, filler (5g of pregelatinized starch), film-forming material (14g of polyvinyl alcohol, 7g of hydroxypropyl methylcellulose), plasticizer (4g of propylene glycol, phthalate Dibutyl formate 4g), flavoring agent (aspartame 1g).
[0032] Preparation:
[0033] 1) Dissolve the film-forming materials (PVA and hydroxypropyl methylcellulose) with 80mL deionized water, and remove the air bubbles to obtain a uniform viscous liquid;
[0034] 2) Disperse propylene glycol, dibutyl phthalate, aspartame, and pregelatinized starch with 60mL of absolute ethanol to form a dispersion;
[0035] 3) Add the dispersion liquid in step 2) to the viscous liquid in step 1), and add dextrooxypyridine to disperse evenly, and then let it stand to remove air bubbles;
[0036] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 65cm / min, then dry at about 75°C, and peel off.
Embodiment 2
[0038] Prescription: D-hydroxypyridamine 55g, polyvinyl alcohol 18g, hydroxypropyl methylcellulose 18g, propylene glycol 2g, dibutyl phthalate 6g, xylitol 1g.
[0039] Preparation:
[0040] 1) Dissolve the film-forming materials (PVA and hydroxypropyl methylcellulose) with 70mL deionized water, and remove the air bubbles to obtain a uniform viscous liquid;
[0041] 2) Disperse propylene glycol, xylitol, and dibutyl phthalate with 50 mL of absolute ethanol to form a dispersion;
[0042] 3) Add the dispersion liquid in step 2) to the viscous liquid in step 1), and add dextrooxypyridine to disperse evenly, and then let it stand to remove air bubbles;
[0043] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 50cm / min, then dry at about 65°C, and peel off.
Embodiment 3
[0045] Prescription: 52g of dextropyridine, 8g of microcrystalline cellulose, 15g of polyvinyl alcohol, 10g of sodium carboxymethylcellulose, 5g of pullulan, 6g of glycerin, 3g of triethyl citrate, 1g of sucralose .
[0046] Preparation:
[0047] 1) Dissolve polyvinyl alcohol, sodium carboxymethyl cellulose, and pullulan in 100 mL of deionized water, and remove air bubbles to obtain a uniform viscous liquid;
[0048] 2) Disperse glycerin, microcrystalline cellulose, sucralose, and triethyl citrate with 50mL of absolute ethanol to form a dispersion;
[0049] 3) Add the dispersion liquid in step 2) to the viscous liquid in step 1), and add dextrooxypyridine to disperse evenly, and then let it stand to remove air bubbles;
[0050] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 80cm / min, then dry at about 85°C, and peel off.
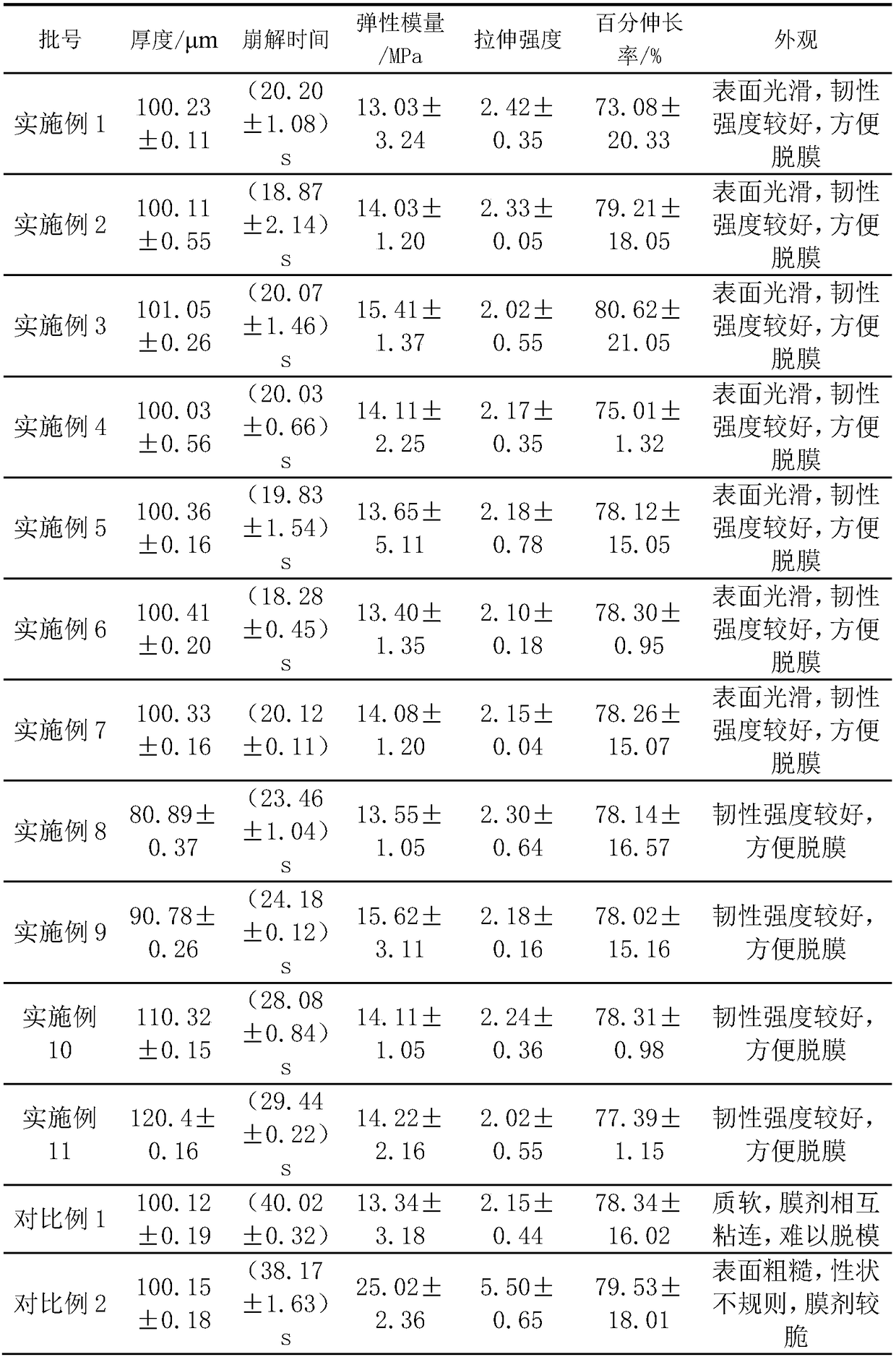
[0051] Referring to the preparation method of Example 1, run Examples 4-17 according to the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com