Method for differentiating inducible pluripotent stem cells into testicular interstitial cells by small molecule induction
A technology of pluripotent stem cells and Leydig cells, applied in the field of Leydig cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the cultivation method of human-derived inducible pluripotent stem cell iPSC
[0035] Inoculate the iPSCs derived from the reprogramming of cloned human urine cells on a 6-well plate that has been incubated with 1% Matrigel (incubate at least 37°C for more than 1 hour), culture in a 37°C carbon dioxide incubator, and invert every day The growth status was observed under a microscope, and fresh E8 medium was replaced in full every day. Once differentiated cells were found, they were picked out in time and passaged every 6 days at a dilution ratio of 6:1. In order to reduce the damage to iPSCs, use 0.25% EDTA for 3-5 minutes during passaging. When the edge of the colony begins to roll up and is about to leave the bottom of the culture dish, wash it once with deionized PBS, and then wash it with 1 Gently pipette mL of E8 medium (no more than 10 times), collect the cells and inoculate them on a new 6-well plate that has been pretreated with 1% Matrigel in mas...
Embodiment 2
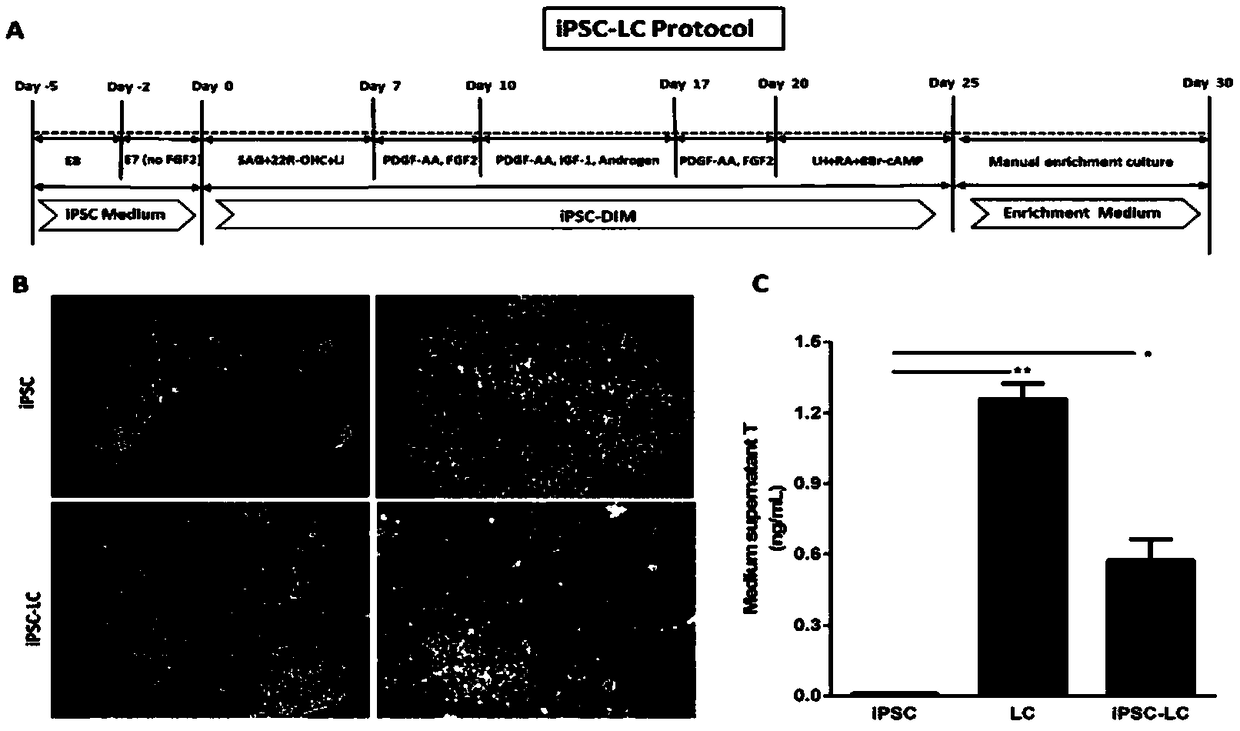
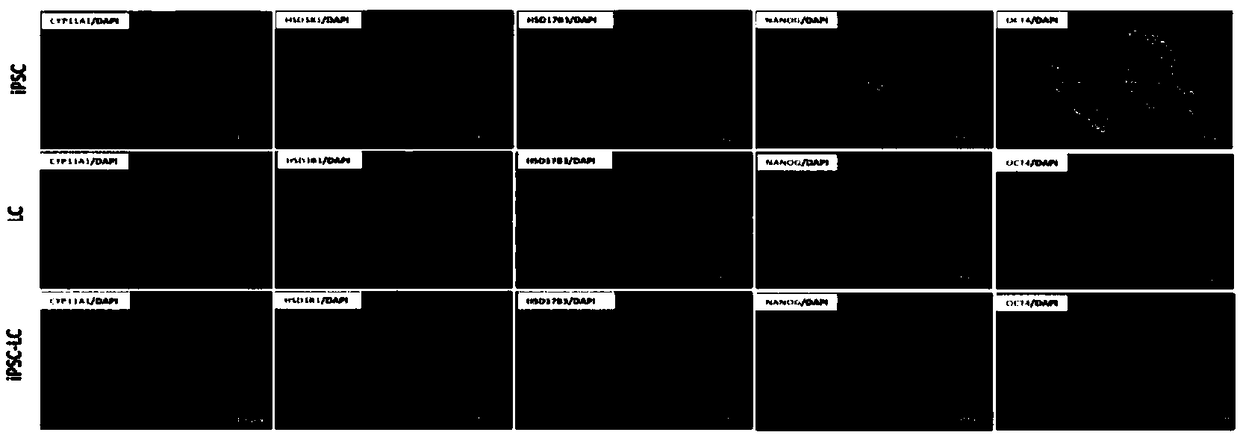
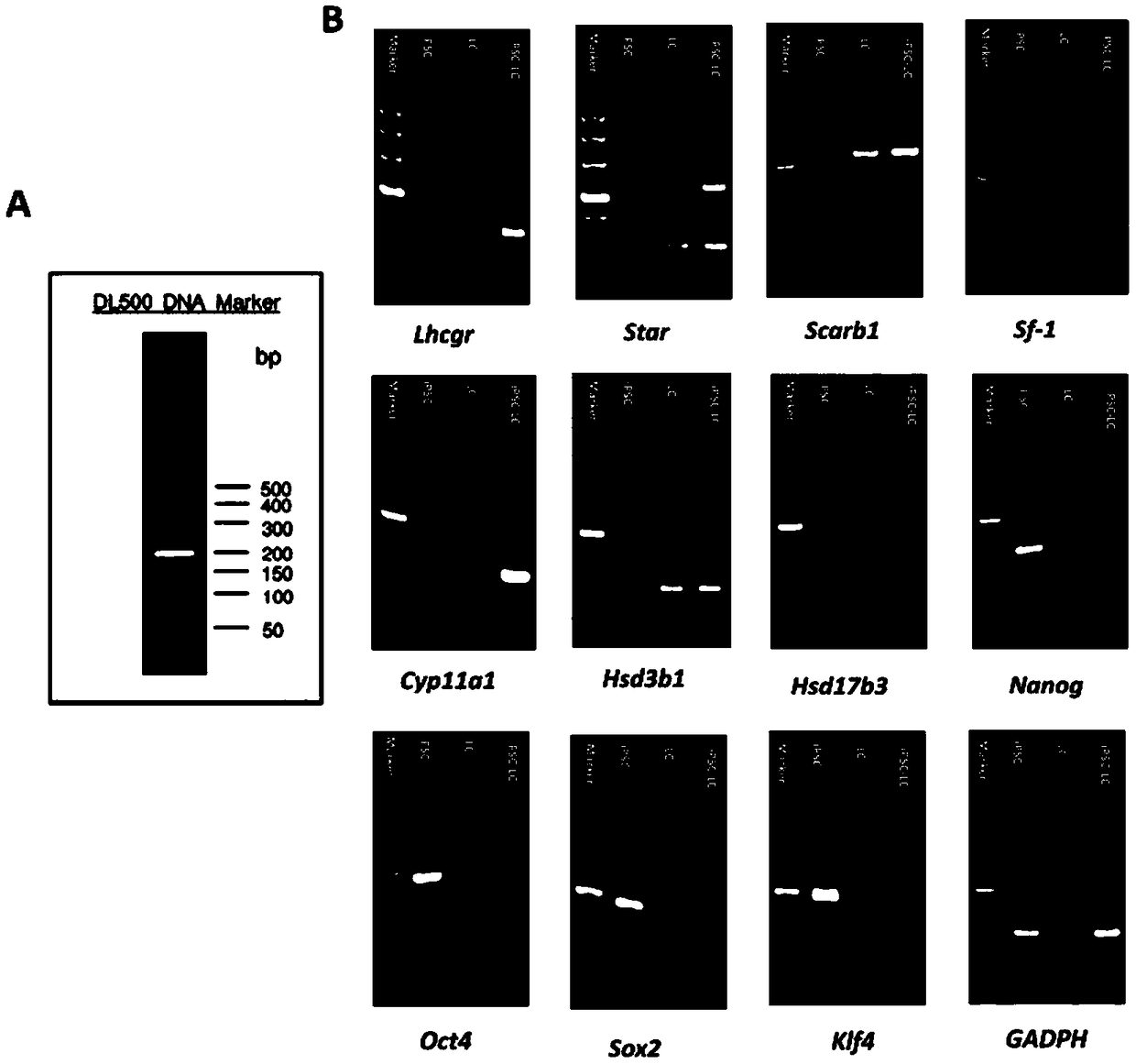
[0042] Example 2: Method for Inducing Human-derived Inducible Pluripotent Stem Cells iPSCs to Differentiate into Leydig Cells iPSC-LCs
[0043] Implementation process: when the clonal mass-like iPSCs cultured in Example 1 reached 70% confluence, this time point was defined as day -2, at which time the cell culture medium was replaced with E7 medium without bFGF for culture, pretreatment 2 days, define this time point as the 0th day. Then the medium was changed into differentiation medium iPSC-DIM: DMEM / F12, 1% bovine serum albumin BSA by volume, 5mM ITS and 5 ng / mL LH. From day 0 to 7, add 0.2 μM SAG, 5 μM 22R-OHC, and 5 mMLi to the differentiation medium for early differentiation induction. From day 7 to 10, add 5 ng / mL PDGF-AA and 5 ng / mLFGF2 to the differentiation medium for early pro-proliferation induction. From day 10 to 17, add 5 ng / mL PDGF-AA, 5 nMIGF-1, and 10 μM Androgen to the differentiation medium for induction of metaphase differentiation. From day 17 to 20, 1...
Embodiment 3
[0045] Embodiment 3: the method for measuring serum testosterone content by radioimmunoassay
[0046] Said sample refers to the cell sample differentiated and formed in Example 2.
[0047] Prepare TBS-G solution: 1 g gelatin, 4.4 g Trizma HCl, 2.65 g Trizma Base, 5.84 g NaCl and 1 g Na Azide are dissolved in 1 L double distilled water. Add 500 μL of TBS-G to the labeled TC tube for complete radioactivity measurement without activated carbon adsorption. Add 500 μL TBS-G to the labeled NBS tube used to measure non-specific binding value without adding antibody. Add 200 µL of TBS-G and 100 µL of sample in a labeled sample tube. Add 300 μL of standards of different concentrations to the tube marked with the standard tube: 10-2000 pg / 100 μL, 8 concentrations. Add 200 μL of testosterone antibody to each tube except TC tube and NBS tube. Add 300 μL Tracer to each tube, that is, H-testosterone-TBS-G solution, shake, and leave overnight at 4°C. Except for TC tubes, add 200 μL of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com