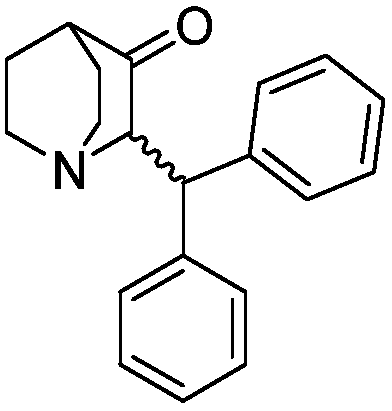
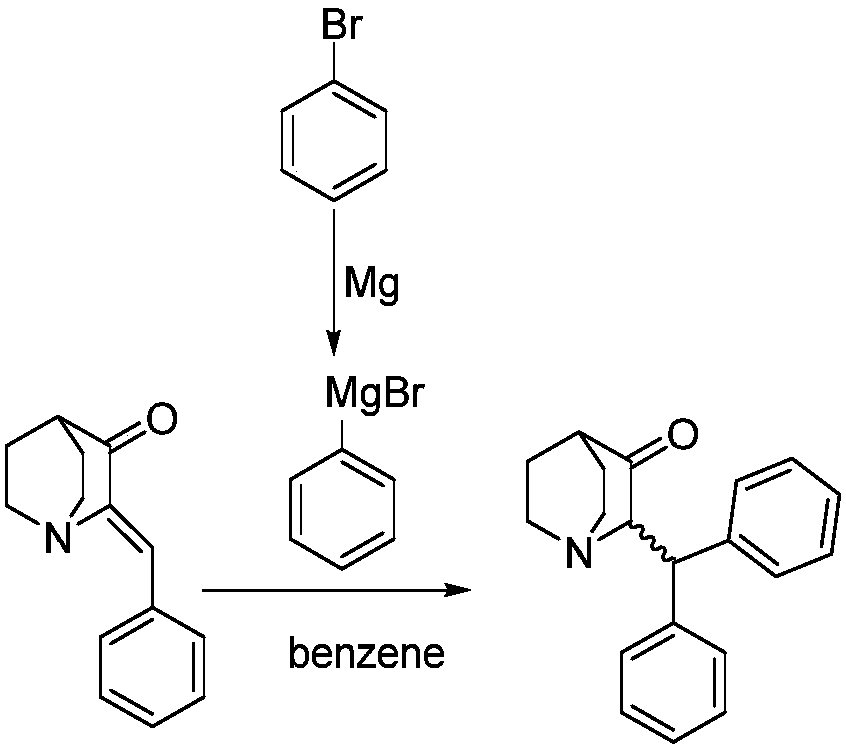
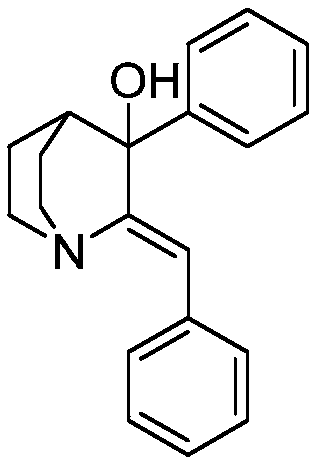
Method of synthesizing benzhydryl quinuclidone through Michael addition
A technology of benzhydrylquinuclidine ketone and benzylidene quinine is applied in the field of organic synthesis, and can solve the problems of environmental protection pressure, undisclosed yield, unfriendly environment and the like in scaled production, and achieve no environmental protection pressure, Low price and low environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh (Z)-2-benzylidene quinine-3-one (9.0g) into a 250mL beaker, add tetrahydrofuran (110mL), stir and dissolve to obtain solution A, and transfer to the dropping funnel. Add 2.0M phenylmagnesium chloride tetrahydrofuran solution (36mL) into a 250mL three-necked flask, add cuprous bromide (602mg), cool down to -5°C under nitrogen protection, add solution A dropwise, and return to 25°C naturally after adding, react for 15 hours, Slowly pour the reaction solution into saturated ammonium chloride solution (40mL), stir for 10 minutes, separate the liquids, extract the aqueous phase twice with ethyl acetate, combine the organic phases, wash with water, wash with saturated sodium chloride solution, and dry over anhydrous sodium sulfate , concentrated in vacuo to obtain a crude product, which was crystallized from absolute ethanol to obtain 2-(benzhydryl)quinuclidin-3-one (10.5g), yield: 85%, HPLC: 99.6%. 1 HNMR (400MHz, CDCl 3 ): δ (ppm) = 7.394-7.133, (m, 10H, Ar-H), 4.524...
Embodiment 2
[0035] Weigh (Z)-2-benzylidene quinine-3-one (10.0g) into a 250mL beaker, add tetrahydrofuran (80mL), stir and dissolve to obtain solution A, and transfer to the dropping funnel. Add 2.0M phenylmagnesium chloride tetrahydrofuran solution (28mL) into a 250mL three-necked flask, add cuprous bromide (674mg), cool down to -5°C under nitrogen protection, add solution A dropwise, and return to 25°C naturally after adding, react for 12 hours, Slowly pour the reaction solution into saturated ammonium chloride solution (45mL), stir for 10 minutes, separate the liquids, extract the aqueous phase twice with ethyl acetate, combine the organic phases, wash with water, wash with saturated sodium chloride solution, and dry over anhydrous sodium sulfate , concentrated in vacuo to obtain the crude product, crystallized with absolute ethanol to obtain 2-(benzhydryl)quinuclidin-3-one (10.9g), yield: 80%, HPLC: 99.2%, NMR and MS data were the same Example 1.
Embodiment 3
[0037] Weigh (Z)-2-benzylidene quinine-3-one (10.0 g) into a 500 mL beaker, add tetrahydrofuran (200 mL), stir and dissolve to obtain solution A, and transfer to the dropping funnel. Add 2.0M phenylmagnesium chloride tetrahydrofuran solution (70mL) into a 500mL three-necked flask, add cuprous bromide (674mg), cool down to -5°C under nitrogen protection, add solution A dropwise, return to 25°C naturally after adding, and react for 24 hours. Slowly pour the reaction solution into saturated ammonium chloride solution (55mL), stir for 10 minutes, separate the liquids, extract the aqueous phase twice with ethyl acetate, combine the organic phases, wash with water, wash with saturated sodium chloride solution, and dry over anhydrous sodium sulfate , concentrated in vacuo to obtain the crude product, crystallized with absolute ethanol to obtain 2-(benzhydryl)quinuclidin-3-one (11.2g), yield: 82%, HPLC: 99.2%, NMR and MS data were the same Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com