Bioactive peptide kss1b and preparation method and application thereof
A kss1b, biologically active peptide technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of high technical threshold, inability to talk about activity, and low degree of product homogeneity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
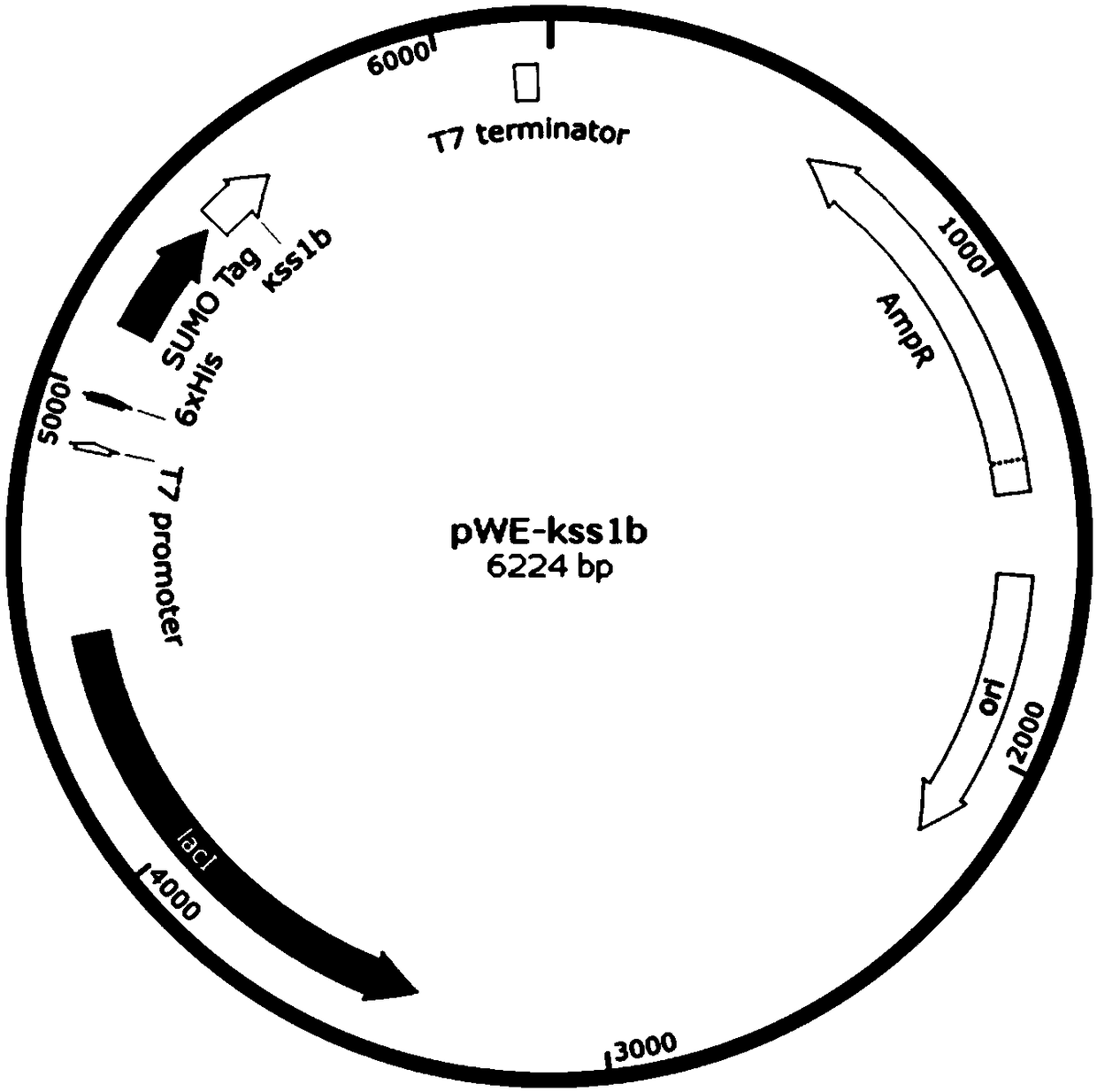
[0044] Construction and use of embodiment 1 expression vector pWE-kss1b
[0045] Using the commercialized pET-43a vector (purchased from Novagen) as a template, a PCR-based seamless cloning method was used to obtain the target plasmid. To put it simply, using the homologous sequence (Tm>60 degrees) between the fragment and the vector, using a commercially available high-fidelity DNA polymerase, the target gene is integrated into the relevant vector by PCR.
[0046] PCR system: 1× buffer, 10pmol primers, 0.5mM dNTP, 1mM MgSO 4 , 1U DNA polymerase and 1ng-60ng template DNA.
[0047] The PCR reaction conditions were: pre-denaturation at 94°C for 2 min; denaturation at 98°C for 10 s, annealing at 60°C for 10 s, extension at 68°C (2 kb / min), a total of 30 cycles.
[0048] The original NusA sequence was replaced by the common PCR-based seamless cloning method in the art, and then the sequence encoding SUMO (SEQ ID NO.2) was inserted downstream of 6×His, and finally the sequence en...
Embodiment 2
[0065] Example 2 Induced Expression of Expression Plasmid pWE-kss1b
[0066] Using the conventional calcium chloride transformation method, the expression plasmid was transformed into E. coli SHuffle TM , and then spread it on ampicillin-resistant (100 μg / mL ampicillin) LB solid medium, and place it upside down at 37° C. for 16 hours. On the next day, pick 1-2 single colonies, inoculate them in 5 mL of ampicillin-resistant 505 culture solution, and cultivate them at 37°C and 220 rpm for 8 hours (this step is the enrichment of the seed medium). Transfer 5 mL of seed culture medium into fresh 500 mL of 5051 auto-induction (100 μg / mL ampicillin) culture solution, and continue culturing at 30° C. and 220 rpm. Choose SHuffle TM strain because it can provide intracellular oxidative conditions that promote the folding of disulfide bonds.
[0067] Among them, the 5051 auto-induction culture medium can be configured with reference to the document Studier, F.W. "Protein Production by...
Embodiment 3
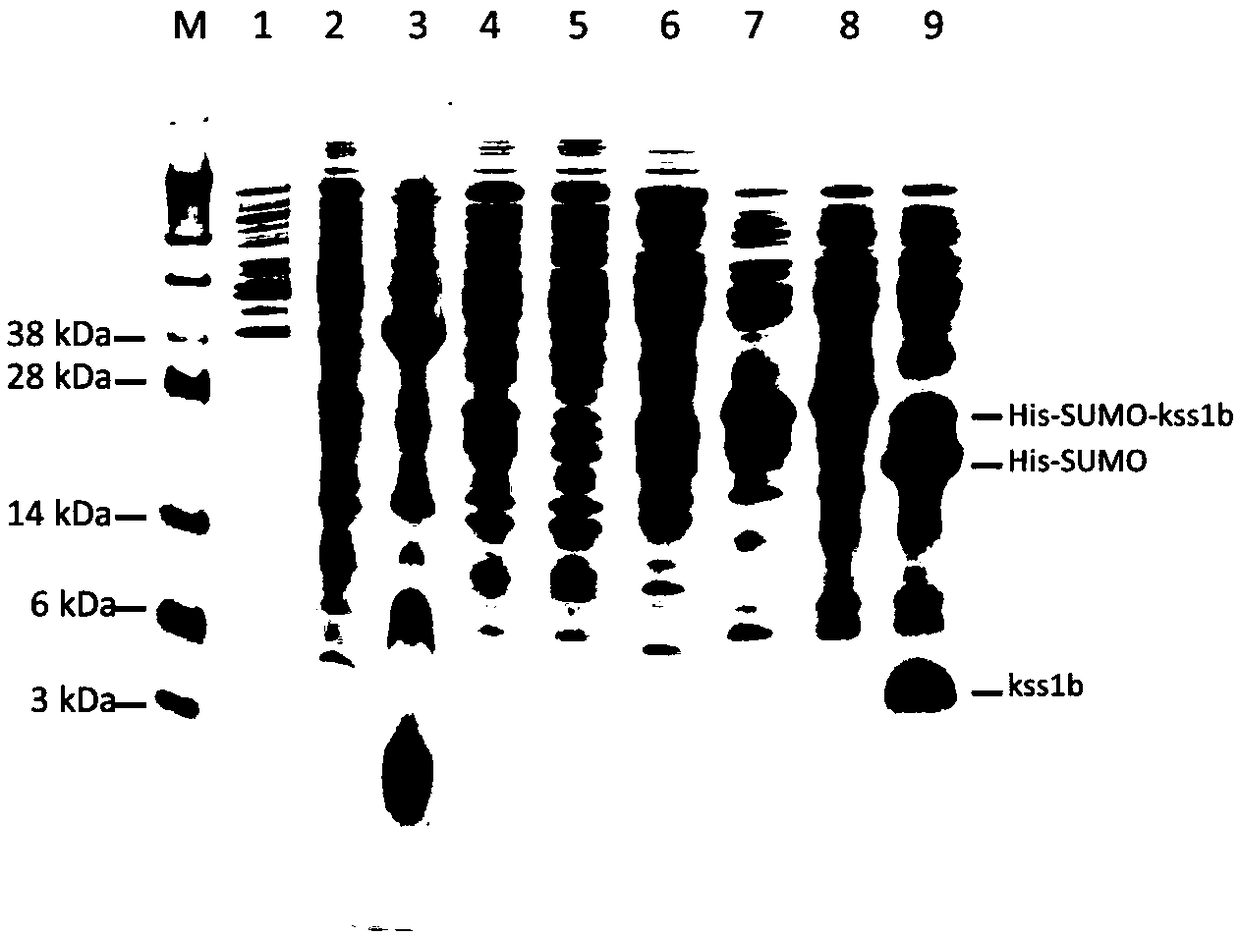
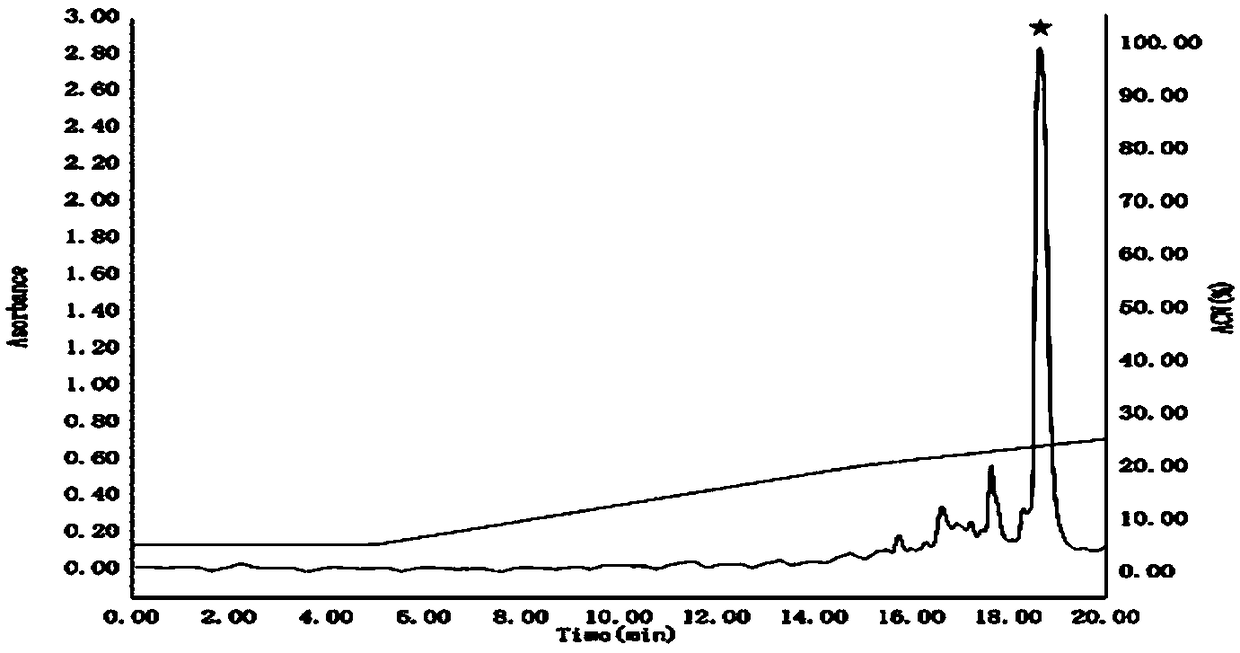
[0068] Example 3 Preliminary separation and purification of kss1b fusion protein and protein kinase digestion
[0069] The thallus that has been induced in Example 2 was centrifuged at 4500 g for 10 minutes to obtain the thallus. Then add an appropriate volume of PBS buffer to resuspend the bacteria, followed by a high-pressure homogenizer for cyclic disruption. After the crushing is completed, centrifuge at 13000rpm for 40 minutes to obtain supernatant and precipitate. The supernatant was purified by nickel column affinity chromatography, and the PBS solution eluate of 200mM imidazole was collected, concentrated and desalted by a 10kDa pore size ultrafiltration tube, and the tube solution in the ultrafiltration tube was collected and an appropriate dose of ULP1 kinase was added (for releasing kss1b), digested overnight at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com